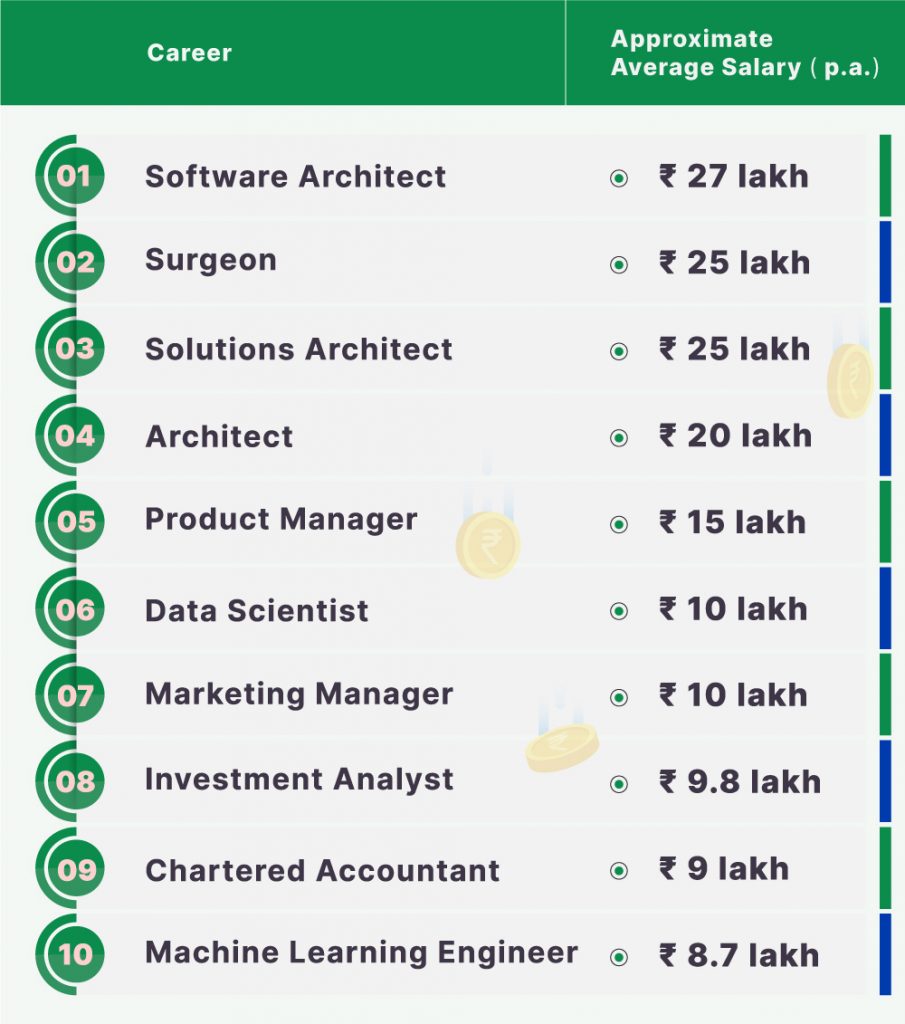
Build an Atom: A Simple Step-by-Step Guide
<!DOCTYPE html>
Have you ever wondered how the building blocks of matter come together? Understanding how to build an atom is a fascinating journey into the heart of chemistry and physics. Whether you're a student, educator, or science enthusiast, this guide will walk you through the process step-by-step. By the end, you'll have a clear understanding of atomic structure and how subatomic particles like protons, neutrons, and electrons work together. Let’s dive in! (atom structure, subatomic particles, science education)
Understanding the Basics of Atomic Structure

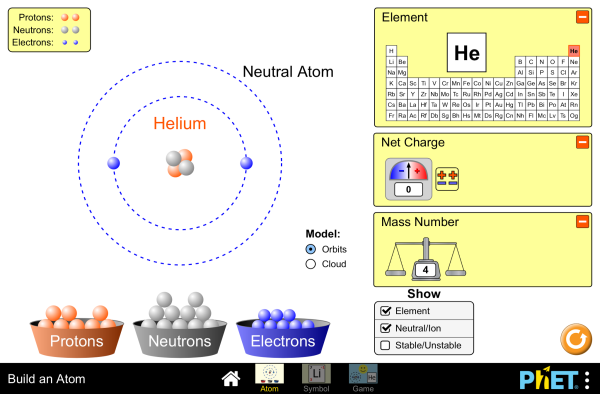
Before we start building an atom, it’s essential to grasp the basics. An atom consists of a nucleus surrounded by electrons. The nucleus contains protons and neutrons, while electrons orbit around it. Protons carry a positive charge, electrons carry a negative charge, and neutrons are neutral. The number of protons determines the atomic number and defines the element. (atomic structure, protons, neutrons, electrons)
Key Components of an Atom
- Protons: Positively charged particles in the nucleus.
- Neutrons: Neutral particles in the nucleus.
- Electrons: Negatively charged particles orbiting the nucleus.
Step-by-Step Guide to Building an Atom

Now that you understand the basics, let’s build an atom step-by-step. We’ll use hydrogen as an example since it’s the simplest element. (build an atom, hydrogen atom)
Step 1: Identify the Element
Choose the element you want to build. For this guide, we’ll build a hydrogen atom. Hydrogen has 1 proton and 1 electron, with no neutrons. (hydrogen atom, atomic number)
Step 2: Create the Nucleus
Start by placing 1 proton in the center to form the nucleus. Since hydrogen has no neutrons, the nucleus only contains the proton. (atom nucleus, protons)
Step 3: Add Electrons
Place 1 electron in the first energy level (or shell) around the nucleus. Electrons orbit the nucleus in specific energy levels. (electron orbit, energy levels)
📌 Note: Electrons fill energy levels in a specific order, starting from the lowest energy level.
Step 4: Verify the Atomic Structure
Double-check your atom. Hydrogen should have 1 proton in the nucleus and 1 electron orbiting it. This completes the basic structure of a hydrogen atom. (atomic structure, hydrogen atom)
Checklist for Building an Atom

- Identify the element and its atomic number.
- Place the correct number of protons in the nucleus.
- Add neutrons to the nucleus (if applicable).
- Arrange electrons in energy levels around the nucleus.
- Verify the atomic structure matches the element’s properties.
By following these steps, you can build any atom, from hydrogen to more complex elements. Understanding atomic structure is crucial for anyone studying chemistry or physics. (atomic structure, chemistry, physics)
What is the role of protons in an atom?
+Protons are positively charged particles located in the nucleus. They determine the atomic number and define the element. (protons, atomic number)
Why do electrons orbit the nucleus?
+Electrons orbit the nucleus due to electromagnetic forces. They occupy specific energy levels based on their energy. (electron orbit, energy levels)
Can an atom exist without neutrons?
+Yes, some atoms like hydrogen can exist without neutrons. However, most elements have neutrons in their nucleus. (neutrons, atomic structure)
Building an atom is a fundamental concept in science that helps us understand the world around us. Whether you're preparing for a class or simply curious about how matter is structured, this guide provides a clear and concise roadmap. Remember, practice makes perfect, so try building atoms of different elements to reinforce your understanding. Happy learning! (build an atom, science education, atomic structure)