Cl Element: Protons, Neutrons, Electrons Explained
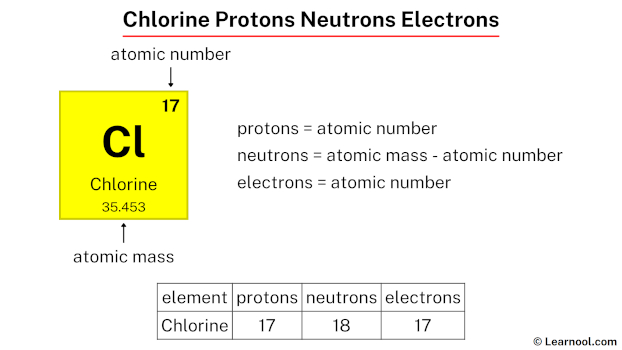
Chlorine (Cl) is a fascinating element with a unique atomic structure that plays a crucial role in various industries, from water purification to chemical manufacturing. Understanding its protons, neutrons, and electrons is essential for anyone studying chemistry or exploring its applications. In this post, we’ll break down the atomic components of chlorine, explain their significance, and provide actionable insights for both informational and commercial audiences. Whether you’re a student, researcher, or industry professional, this guide will equip you with the knowledge you need.
What Are Protons, Neutrons, and Electrons in Chlorine (Cl)?

Chlorine, with the atomic number 17, is composed of subatomic particles that define its properties. Here’s a quick overview:
- Protons: Chlorine has 17 protons, determining its atomic number and identity as Cl.
- Neutrons: The most common isotope, Chlorine-35, has 18 neutrons, while Chlorine-37 has 20 neutrons.
- Electrons: In a neutral chlorine atom, there are 17 electrons, balancing the charge of the protons.
These particles work together to give chlorine its unique chemical behavior, such as its high reactivity and ability to form compounds like sodium chloride (table salt). (chlorine properties, atomic structure, chemical behavior)
Why Do Protons, Neutrons, and Electrons Matter in Chlorine?
Understanding these subatomic particles is key to grasping chlorine’s role in various fields. Here’s why they matter:
- Protons: Define chlorine’s identity and its position on the periodic table.
- Neutrons: Influence isotopes and atomic mass, affecting chlorine’s stability in reactions.
- Electrons: Determine its reactivity and bonding capabilities, essential for industrial applications.
📌 Note: Chlorine’s electron configuration (2-8-7) explains its tendency to gain one electron to achieve a stable state, making it highly reactive.
(chlorine isotopes, electron configuration, industrial applications)
Chlorine in Commercial Applications: A Practical Perspective

For commercial audiences, chlorine’s atomic structure directly impacts its utility in industries like:
- Water Treatment: Chlorine’s electrons allow it to kill bacteria and disinfect water effectively.
- Chemical Manufacturing: Its reactivity makes it a key component in producing plastics, solvents, and pharmaceuticals.
- Household Products: Chlorine-based compounds are used in bleach, cleaning agents, and pool sanitizers.
Investing in chlorine-related products or technologies? Understanding its atomic foundation ensures informed decision-making. (water treatment, chemical manufacturing, household products)
| Component | Chlorine-35 | Chlorine-37 |
|---|---|---|
| Protons | 17 | 17 |
| Neutrons | 18 | 20 |
| Electrons | 17 | 17 |

(chlorine isotopes, atomic components, quick reference)
Key Takeaways: Chlorine’s Atomic Structure

- ✅ Chlorine has 17 protons, defining its atomic number.
- ✅ Neutrons vary between 18 (Chlorine-35) and 20 (Chlorine-37), influencing isotopes.
- ✅ 17 electrons balance the charge and determine reactivity.
- ✅ Chlorine’s structure is vital for its applications in water treatment, manufacturing, and household products.
(key takeaways, atomic structure, chlorine applications)
Chlorine’s protons, neutrons, and electrons are the building blocks of its unique properties and applications. Whether you’re studying chemistry or exploring commercial uses, understanding these components provides a solid foundation. From disinfecting water to producing essential chemicals, chlorine’s atomic structure is at the heart of its versatility. Use the checklist above to reinforce your knowledge and apply it to your specific needs.
(chlorine uses, atomic foundation, practical applications)
How many protons does chlorine have?
+
Chlorine has 17 protons, which defines its atomic number and identity on the periodic table.
What is the difference between Chlorine-35 and Chlorine-37?
+
Chlorine-35 has 18 neutrons, while Chlorine-37 has 20 neutrons. Both have the same number of protons and electrons but differ in atomic mass.
Why is chlorine reactive?
+
Chlorine has 17 electrons arranged in a 2-8-7 configuration. It seeks to gain one more electron to achieve a stable state, making it highly reactive.



