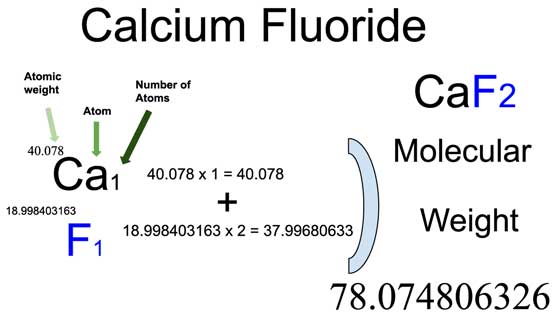
Moles to Molecules: Simple Conversion Guide
Converting moles to molecules is a fundamental skill in chemistry, essential for understanding the relationship between macroscopic and microscopic quantities. Whether you're a student, researcher, or industry professional, mastering this conversion ensures accuracy in calculations and experiments. This guide simplifies the process, providing step-by-step instructions and practical tips to make moles to molecules conversion effortless. (moles to molecules conversion, chemistry basics, Avogadro's number)
Understanding Moles and Molecules: The Basics

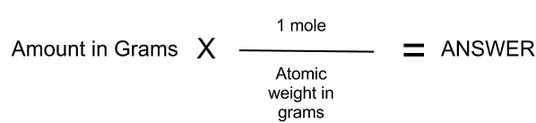
Before diving into the conversion process, it’s crucial to grasp the concepts of moles and molecules. A mole is a unit used to measure the amount of a substance, containing approximately 6.022 x 10²³ particles (Avogadro’s number). A molecule, on the other hand, is the smallest unit of a compound that retains its chemical properties. Understanding these definitions lays the foundation for accurate conversions. (mole definition, molecule definition, Avogadro’s number)
Why Convert Moles to Molecules?
Converting moles to molecules is vital for various applications, including stoichiometry, reaction planning, and material synthesis. It bridges the gap between theoretical calculations and practical laboratory work, ensuring precise measurements. (stoichiometry, chemical reactions, laboratory calculations)
Step-by-Step Guide: Moles to Molecules Conversion

Follow these simple steps to convert moles to molecules seamlessly:
- Step 1: Identify the Number of Moles – Determine the quantity of moles you need to convert.
- Step 2: Apply Avogadro’s Number – Multiply the number of moles by 6.022 x 10²³ to find the number of molecules.
- Step 3: Verify Units – Ensure the final result is in molecules, confirming the conversion is correct.
📌 Note: Always double-check your calculations to avoid errors in experimental setups. (Avogadro’s number, conversion steps, unit verification)
Example Conversion
Let’s convert 2 moles of water (H₂O) to molecules:
| Step | Calculation | Result |
|---|---|---|
| 1 | 2 moles × 6.022 × 10²³ molecules/mole | 1.2044 × 10²⁴ molecules |

This example demonstrates how straightforward the conversion process can be. (example calculation, water molecules, conversion table)
Practical Tips for Accurate Conversions
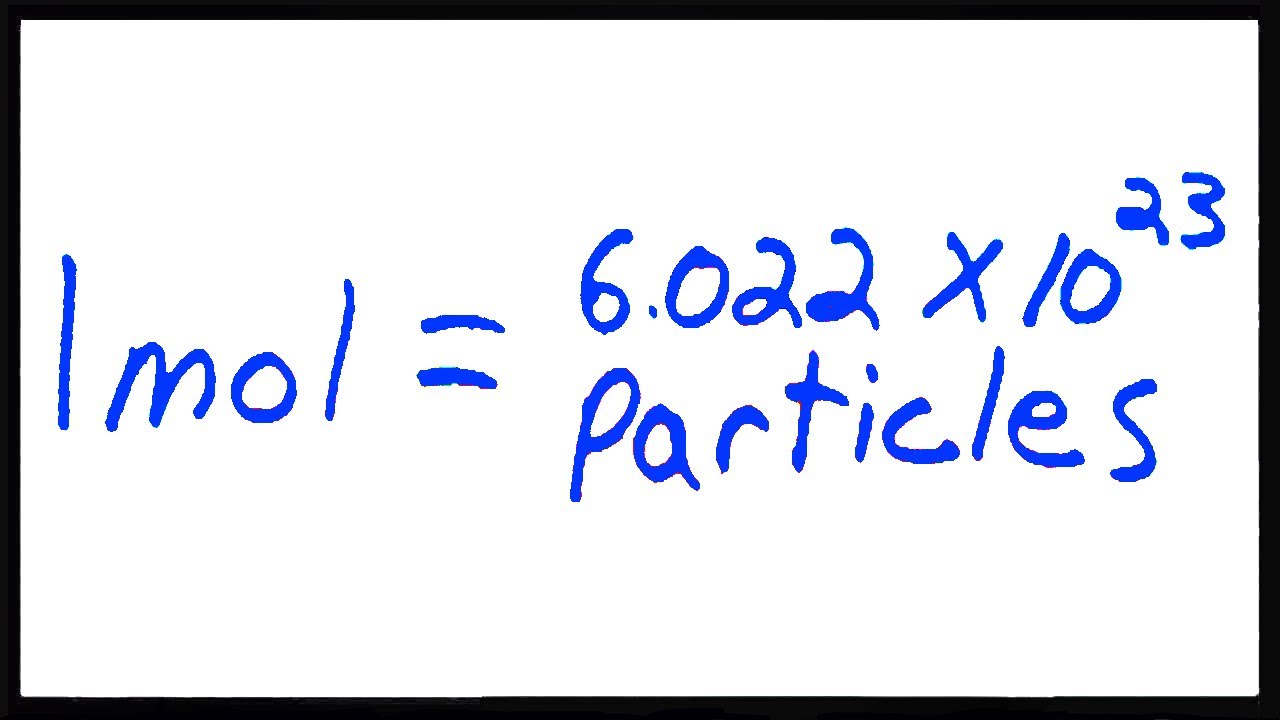
To ensure precision in your conversions, consider these tips:
- Use a Calculator – Avoid manual errors by using a scientific calculator for large numbers.
- Check Significant Figures – Maintain consistency in significant figures throughout your calculations.
- Understand Context – Tailor your conversion to the specific chemical or scenario you’re working with.
✨ Note: Practice regularly to build confidence in handling complex conversions. (calculation tips, significant figures, practical advice)
Tools and Resources for Easy Conversion

Leverage these tools to simplify moles to molecules conversion:
- Online Converters – Use reliable online tools for quick and accurate results.
- Chemistry Software – Explore software like ChemCalc for advanced calculations.
- Reference Tables – Keep Avogadro’s number and other constants handy for quick reference.
These resources save time and minimize errors, making conversions more efficient. (online tools, chemistry software, reference tables)
Mastering moles to molecules conversion is a cornerstone of chemistry, enabling precise calculations and experiments. By following this guide, you’ll gain the skills and confidence to handle conversions with ease. Remember to practice regularly, use reliable tools, and always verify your results for accuracy. Whether you’re in the lab or the classroom, this knowledge will serve as a valuable asset in your scientific journey. (chemistry skills, precise calculations, scientific journey)
What is Avogadro’s number?
+
Avogadro’s number is 6.022 x 10²³, representing the number of particles in one mole of a substance. (Avogadro’s number, mole definition)
Why is moles to molecules conversion important?
+
It bridges macroscopic and microscopic quantities, ensuring accuracy in chemical calculations and experiments. (chemical calculations, experimental accuracy)
Can I use online tools for conversion?
+
Yes, reliable online converters can simplify the process, but understanding the manual method is essential. (online converters, manual conversion)