Unveiling the Unique Blocks of the Periodic Table
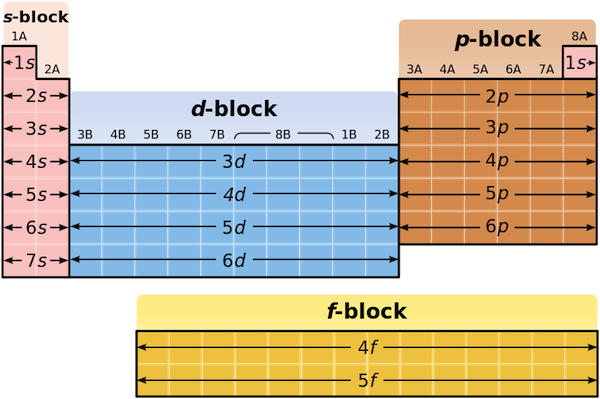
The periodic table, a cornerstone of chemistry, is a treasure trove of elements, each with its unique properties and applications. Understanding the blocks of the periodic table is crucial for anyone delving into the world of chemistry, whether for academic purposes or industrial applications. Let’s explore these blocks in detail, uncovering their significance and how they shape our understanding of the elements.
Understanding the Blocks of the Periodic Table

The periodic table is divided into four main blocks based on the electron configuration of the elements: s-block, p-block, d-block, and f-block. Each block represents a specific orbital where the valence electrons reside, influencing the element’s chemical behavior.
s-Block Elements: The Alkali and Alkaline Earth Metals
The s-block elements are found in the first two groups of the periodic table. These include the alkali metals (Group 1) and alkaline earth metals (Group 2). Known for their high reactivity, these metals are soft, have low melting points, and readily lose electrons to form +1 and +2 ions, respectively.
- Key Properties: High reactivity, low density, and excellent conductors of electricity.
- Examples: Sodium (Na), Potassium (K), Calcium (Ca), Magnesium (Mg).
📌 Note: s-block elements are essential in biological processes and industrial applications, such as batteries and alloys.
p-Block Elements: The Diverse Group
The p-block elements occupy the right side of the periodic table (Groups 13–18). This block is the most diverse, containing metals, nonmetals, and metalloids. Elements in this block have their valence electrons in the p-orbital, leading to varied chemical properties.
- Key Properties: Wide range of physical states, variable reactivity, and ability to form multiple bonds.
- Examples: Carbon ©, Oxygen (O), Silicon (Si), Chlorine (Cl).
d-Block Elements: The Transition Metals
The d-block elements are the transition metals, positioned in the middle of the periodic table (Groups 3–12). These elements are known for their metallic properties, high melting points, and ability to form multiple oxidation states.
- Key Properties: Hardness, high melting points, and catalytic activity.
- Examples: Iron (Fe), Copper (Cu), Zinc (Zn), Gold (Au).
📌 Note: Transition metals are widely used in construction, electronics, and catalysis due to their strength and versatility.
f-Block Elements: The Lanthanides and Actinides
The f-block elements consist of the lanthanides and actinides, placed separately at the bottom of the periodic table. These elements have their valence electrons in the f-orbital, making them highly complex and often radioactive.
- Key Properties: Radioactivity, high density, and complex electronic structures.
- Examples: Uranium (U), Plutonium (Pu), Lanthanum (La), Cerium (Ce).
Applications of Periodic Table Blocks in Industry
Each block of the periodic table plays a unique role in various industries. For instance:
- s-Block: Used in medicine (e.g., Lithium for bipolar disorder) and energy storage (e.g., Sodium-ion batteries).
- p-Block: Essential in electronics (e.g., Silicon in semiconductors) and life sciences (e.g., Carbon in organic compounds).
- d-Block: Crucial in construction (e.g., Iron in steel) and catalysis (e.g., Platinum in catalytic converters).
- f-Block: Utilized in nuclear energy (e.g., Uranium in reactors) and specialized materials (e.g., Neodymium in magnets).
| Block | Common Uses |
|---|---|
| s-Block | Medicine, Batteries |
| p-Block | Electronics, Life Sciences |
| d-Block | Construction, Catalysis |
| f-Block | Nuclear Energy, Specialized Materials |

Checklist for Understanding Periodic Table Blocks
- Identify the electron configuration of each block (s, p, d, f).
- Recognize the physical and chemical properties associated with each block.
- Explore industrial applications of elements from different blocks.
- Study examples of elements in each block for better understanding.
The periodic table’s blocks provide a structured way to understand the elements and their behaviors. Whether you’re a student, researcher, or industry professional, mastering these blocks is essential for leveraging the power of chemistry in real-world applications.
What are the main blocks of the periodic table?
+The periodic table is divided into four main blocks: s-block, p-block, d-block, and f-block, based on the electron configuration of the elements.
What are s-block elements used for?
+s-block elements are used in medicine, energy storage, and industrial applications due to their high reactivity and conductivity.
Why are d-block elements called transition metals?
+d-block elements are called transition metals because they exhibit properties intermediate between s-block and p-block elements, such as multiple oxidation states and metallic behavior.
periodic table blocks, s-block elements, p-block elements, d-block elements, f-block elements, transition metals, alkali metals, alkaline earth metals, lanthanides, actinides.



