Unlocking Carbon's Electron Arrangement Secrets

Carbon, the backbone of organic chemistry, holds secrets in its electron arrangement that have fascinated scientists for decades. Understanding its electron configuration is crucial for advancements in materials science, nanotechnology, and even climate solutions. This blog delves into the mysteries of carbon’s electron arrangement, exploring its significance, applications, and how it shapes our world. Whether you're a student, researcher, or industry professional, this guide unlocks the science behind carbon’s unique properties, carbon electron arrangement, carbon atomic structure, and carbon bonding principles.
Understanding Carbon’s Electron Configuration

Carbon’s atomic number is 6, meaning it has 6 protons and 6 electrons. Its electron arrangement is 1s² 2s² 2p², which allows it to form four covalent bonds, a key factor in its versatility. This configuration is the foundation of organic compounds, from diamonds to graphene. By mastering carbon’s electron structure, we can harness its potential in innovative technologies like carbon capture and advanced materials.
The Role of Valence Electrons in Carbon Bonding
Carbon’s four valence electrons in the 2s and 2p orbitals enable it to form strong, stable bonds. This property is essential for creating complex molecules such as proteins, DNA, and plastics. Understanding valence electrons in carbon helps in designing new materials and optimizing chemical reactions. (carbon bonding principles, carbon valence electrons, organic chemistry)
Applications of Carbon’s Electron Arrangement
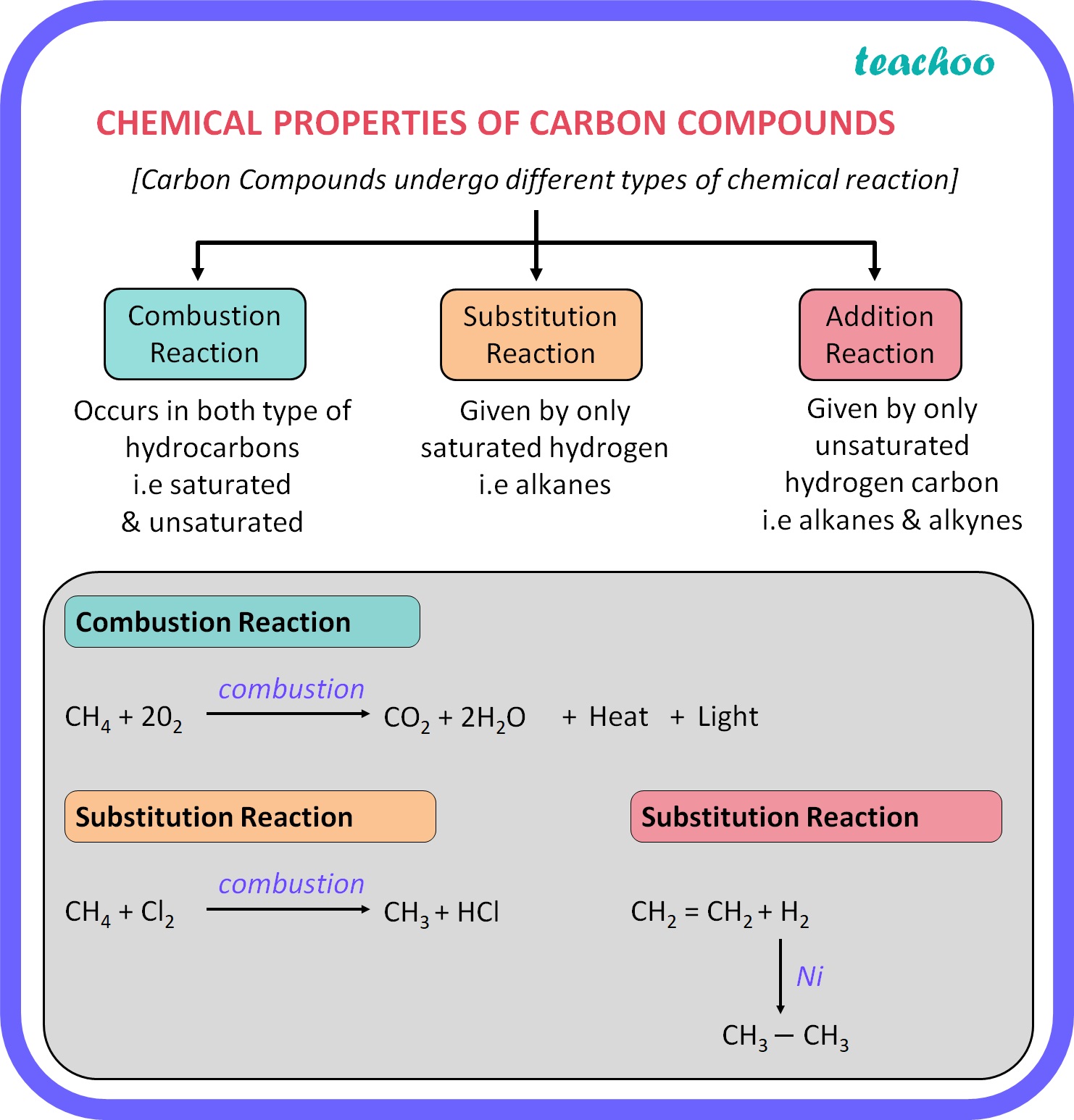
The unique electron configuration of carbon drives its applications across industries. From carbon fiber in aerospace to graphene in electronics, its versatility is unmatched. Below is a table summarizing key applications:
| Application | Description |
|---|---|
| Carbon Fiber | Lightweight, high-strength material used in aerospace and automotive industries. |
| Graphene | Single-layer carbon atoms with exceptional conductivity for electronics. |
| Carbon Capture | Technologies leveraging carbon’s bonding to reduce greenhouse gases. |

Carbon in Nanotechnology
Nanotechnology relies heavily on carbon’s electron arrangement. Fullerenes, nanotubes, and graphene are prime examples of carbon-based nanomaterials. These structures exhibit unique properties like high conductivity and strength, making them ideal for next-gen devices. (carbon nanotechnology, graphene applications, carbon nanomaterials)
💡 Note: Carbon’s ability to form double and triple bonds further enhances its utility in diverse applications.
Carbon’s electron arrangement is a cornerstone of modern science and technology. From its atomic structure to its role in nanotechnology, understanding carbon unlocks endless possibilities. Whether you're exploring carbon electron arrangement for research or industry, this knowledge is invaluable. Dive deeper into carbon atomic structure, carbon bonding principles, and carbon valence electrons to stay ahead in the ever-evolving field of materials science.
What is carbon’s electron configuration?
+
Carbon’s electron configuration is 1s² 2s² 2p², with four valence electrons in the 2s and 2p orbitals.
Why is carbon’s electron arrangement important?
+
It allows carbon to form four strong covalent bonds, making it the basis of organic chemistry and advanced materials.
How is carbon used in nanotechnology?
+
Carbon is used in nanomaterials like graphene and nanotubes due to its unique electron arrangement and properties.



