Electrons in Carbon Atoms: Structure & Function Explained

Carbon atoms are the backbone of organic chemistry, and their unique structure is defined by the arrangement and behavior of their electrons. Understanding the role of electrons in carbon atoms is crucial for grasping how carbon forms the basis of life and numerous materials. This post delves into the electron structure of carbon, its functionality, and its significance in both scientific and commercial applications.
Electron Structure of Carbon Atoms
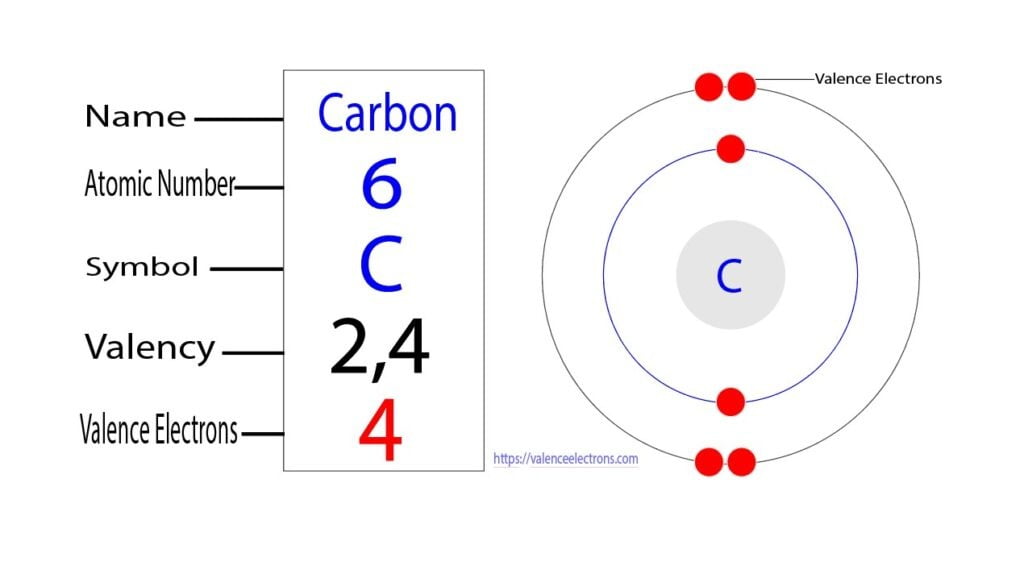
Carbon has an atomic number of 6, meaning it has 6 electrons. These electrons are arranged in energy levels or shells around the nucleus. The electron configuration of carbon is 1s² 2s² 2p², indicating that the first shell holds 2 electrons, and the second shell holds 4 electrons.
Electron Distribution in Carbon
- 1s²: The first shell, closest to the nucleus, is fully occupied with 2 electrons.
- 2s²: The second shell’s s-orbital holds another 2 electrons.
- 2p²: The remaining 2 electrons occupy the p-orbital of the second shell.
📌 Note: The 2p orbital is only partially filled, allowing carbon to form covalent bonds with other atoms.
Function of Electrons in Carbon Atoms
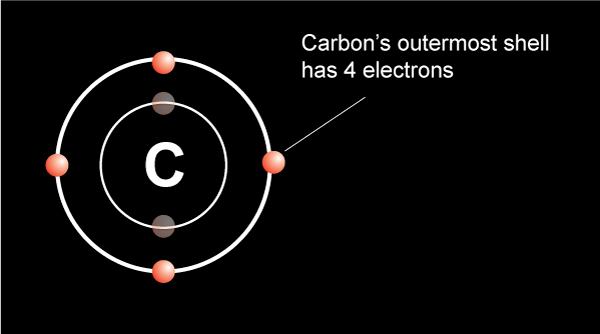
The electrons in carbon atoms play a pivotal role in determining its chemical behavior. Carbon’s ability to form four covalent bonds is due to the four electrons in its outer shell (2s² 2p²). This property makes carbon highly versatile in forming complex molecules.
Key Functions of Carbon Electrons
- Bond Formation: Electrons in the 2p orbital participate in bonding, enabling carbon to form stable molecules like methane (CH₄) and ethylene (C₂H₄).
- Hybridization: Carbon atoms can undergo sp³, sp², or sp hybridization to form different molecular geometries.
- Electron Sharing: Carbon shares electrons with other atoms, creating a network of covalent bonds essential for organic compounds.
| Hybridization Type | Geometry | Example |
|---|---|---|
| sp³ | Tetrahedral | Methane (CH₄) |
| sp² | Trigonal Planar | Ethylene (C₂H₄) |
| sp | Linear | Acetylene (C₂H₂) |

Commercial and Scientific Applications
The unique electron structure of carbon atoms has led to groundbreaking applications in both science and industry. From graphene to carbon nanotubes, carbon’s versatility is unmatched.
Applications of Carbon-Based Materials
- Graphene: A single layer of carbon atoms arranged in a hexagonal lattice, used in electronics and composites.
- Carbon Fiber: Lightweight and strong, ideal for aerospace and automotive industries.
- Organic Chemistry: Carbon’s bonding ability forms the basis of pharmaceuticals, polymers, and fuels.
📌 Note: Carbon capture technology leverages carbon’s reactivity to mitigate climate change.
Summary and Checklist

Understanding the electron structure and function of carbon atoms is essential for appreciating its role in science and technology. Here’s a quick checklist to recap:
- Carbon has 6 electrons arranged as 1s² 2s² 2p².
- The 2p orbital allows carbon to form four covalent bonds.
- Carbon’s hybridization enables diverse molecular geometries.
- Applications range from graphene to carbon fiber.
Carbon’s electron structure is the foundation of its versatility, making it a cornerstone of both natural and synthetic materials. Whether in organic chemistry, material science, or environmental technology, carbon’s electrons drive innovation and progress.
What is the electron configuration of carbon?
+Carbon’s electron configuration is 1s² 2s² 2p², with 6 electrons distributed across two energy levels.
How does carbon form four bonds?
+Carbon forms four bonds through the sharing of electrons in its 2p orbital, allowing it to achieve a stable octet configuration.
What are some commercial uses of carbon-based materials?
+Commercial uses include graphene in electronics, carbon fiber in aerospace, and carbon compounds in pharmaceuticals.