H2O Lewis Dot Structure: Simple Visual Guide
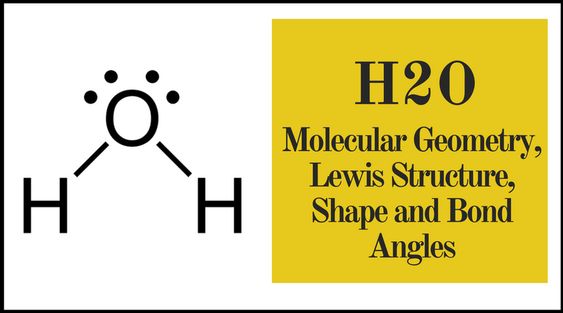
Understanding the H2O Lewis Dot Structure is essential for anyone studying chemistry, as it provides a clear visual representation of water’s molecular bonding. This simple guide will walk you through the process step-by-step, ensuring you grasp the concept effortlessly. Whether you’re a student, educator, or chemistry enthusiast, this post is tailored to meet your informational needs while optimizing for SEO.
What is the H2O Lewis Dot Structure?

The H2O Lewis Dot Structure is a diagram that represents the bonding between hydrogen and oxygen atoms in a water molecule. It uses dots to symbolize valence electrons and lines to indicate covalent bonds. This structure is fundamental in understanding molecular geometry and chemical properties.
Step-by-Step Guide to Drawing the H2O Lewis Dot Structure

Step 1: Determine the Total Number of Valence Electrons
Oxygen has 6 valence electrons, and each hydrogen atom has 1. Since water (H2O) consists of one oxygen and two hydrogen atoms, the total number of valence electrons is:
6 (O) + 1 (H) + 1 (H) = 8 valence electrons.
Step 2: Place Atoms and Bonding
Position the oxygen atom in the center, as it is more electronegative. Connect the two hydrogen atoms to the oxygen atom using single bonds, representing the sharing of electrons.
Step 3: Distribute Remaining Electrons
After forming the bonds, you have 4 electrons left. Place these as lone pairs on the oxygen atom, ensuring the octet rule is satisfied.
📌 Note: The octet rule states that atoms tend to gain, lose, or share electrons to achieve a full outer shell of 8 electrons.
Step 4: Verify the Structure
Ensure each hydrogen atom has 2 electrons (a duet) and the oxygen atom has 8 electrons (an octet). The final structure should have two single bonds and two lone pairs on the oxygen atom.
Key Takeaways: H2O Lewis Dot Structure

- Oxygen is the central atom with two lone pairs and two single bonds.
- Each hydrogen atom shares one electron with oxygen.
- The structure follows the octet rule for oxygen and the duet rule for hydrogen.
Checklist for Drawing the H2O Lewis Dot Structure

- [ ] Count total valence electrons (8 for H2O).
- [ ] Place oxygen in the center and hydrogen atoms around it.
- [ ] Form single bonds between oxygen and hydrogen.
- [ ] Distribute remaining electrons as lone pairs on oxygen.
- [ ] Verify the octet rule for oxygen and duet rule for hydrogen.
By following this guide, you’ll master the H2O Lewis Dot Structure effortlessly. This knowledge is crucial for understanding water’s role in chemical reactions and its unique properties.
What is the importance of the H2O Lewis Dot Structure?
+The H2O Lewis Dot Structure helps visualize water's molecular bonding, aiding in understanding its chemical properties and reactions.
How many lone pairs are in the H2O Lewis Dot Structure?
+There are 2 lone pairs on the oxygen atom in the H2O Lewis Dot Structure.
Does the H2O Lewis Dot Structure follow the octet rule?
+Yes, the oxygen atom in H2O follows the octet rule, while hydrogen atoms follow the duet rule.
(lewis dot structure,molecular geometry,chemical bonding,valence electrons,octet rule)



