HCl + NaOH Reaction: Neutralization Explained Simply
The reaction between hydrochloric acid (HCl) and sodium hydroxide (NaOH) is a classic example of a neutralization reaction, a fundamental concept in chemistry. This reaction is not only fascinating but also highly practical, with applications ranging from industrial processes to everyday household uses. Understanding the HCl + NaOH reaction can help demystify the chemistry behind acid-base interactions, making it an essential topic for students, educators, and professionals alike. Whether you’re looking to deepen your knowledge or find practical solutions, this post will guide you through the process, products, and significance of this chemical reaction.
Understanding the HCl + NaOH Reaction

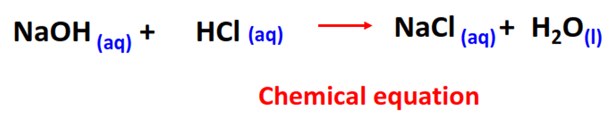
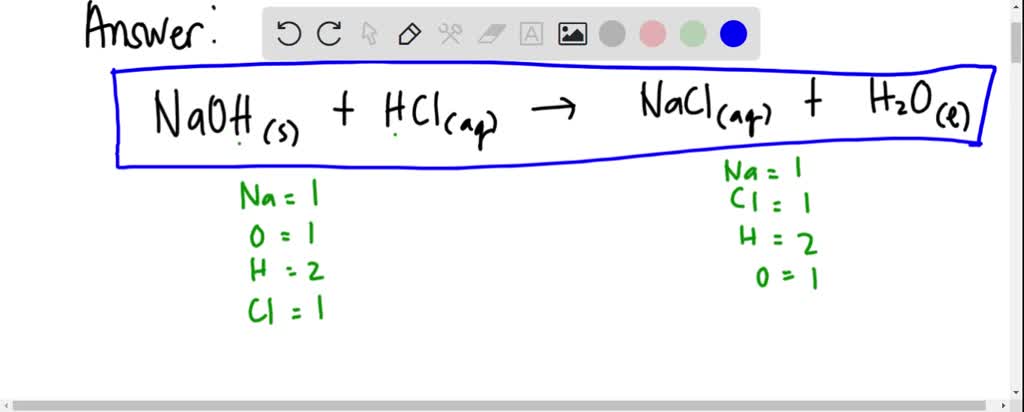
The reaction between hydrochloric acid (HCl) and sodium hydroxide (NaOH) is a prime example of acid-base neutralization. When these two substances mix, they form water (H₂O) and sodium chloride (NaCl), a type of salt. The balanced chemical equation for this reaction is:
HCl + NaOH → H₂O + NaCl
This reaction is not only straightforward but also highly efficient, making it a cornerstone in various chemical processes.
Key Components of the Reaction
- Hydrochloric Acid (HCl): A strong acid commonly used in laboratories and industries.
- Sodium Hydroxide (NaOH): A strong base, also known as caustic soda, widely used in manufacturing and cleaning.
- Water (H₂O): The primary product of the reaction, formed when H⁺ from HCl combines with OH⁻ from NaOH.
- Sodium Chloride (NaCl): Table salt, a neutral compound that remains after the reaction.
💡 Note: The reaction is exothermic, meaning it releases heat. Always handle these chemicals with care and proper safety equipment.
The Neutralization Process
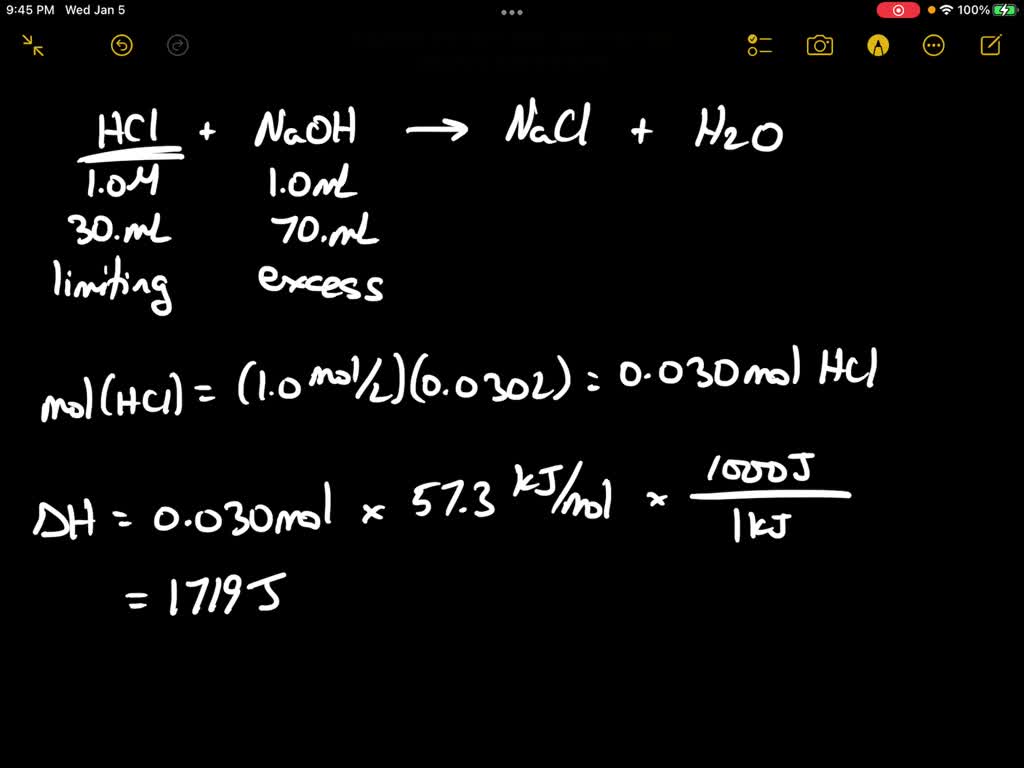
Neutralization occurs when an acid and a base react to form water and a salt. In the case of HCl and NaOH, the H⁺ ion from the acid combines with the OH⁻ ion from the base to produce water, while the remaining Na⁺ and Cl⁻ ions form sodium chloride.
Step-by-Step Breakdown
- Mixing the Solutions: Combine measured amounts of HCl and NaOH in a controlled environment.
- Ion Exchange: H⁺ ions from HCl and OH⁻ ions from NaOH interact.
- Formation of Products: Water (H₂O) and sodium chloride (NaCl) are produced.
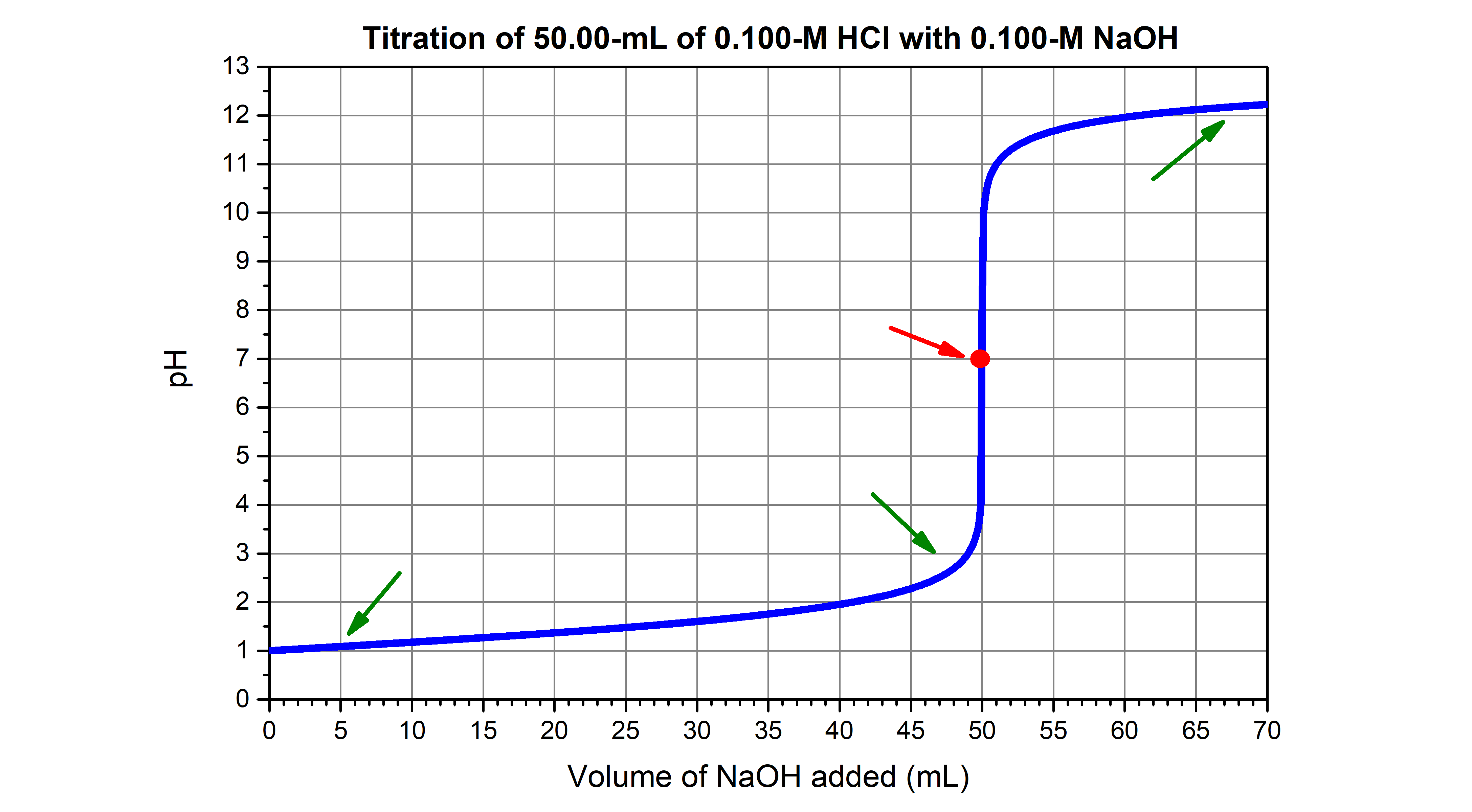
- Completion: The solution becomes neutral, with a pH close to 7.
| Reactants | Products |
|---|---|
| HCl (Acid) | H₂O (Water) |
| NaOH (Base) | NaCl (Salt) |

Practical Applications
- Chemical Manufacturing: Used in producing pharmaceuticals, soaps, and detergents.
- Wastewater Treatment: Neutralizes acidic or basic waste before disposal.
- Household Cleaning: Found in drain cleaners to dissolve clogs.
Safety and Precautions
Working with HCl and NaOH requires caution due to their corrosive nature. Always wear protective gear, including gloves and goggles, and ensure proper ventilation.
⚠️ Note: Never mix these chemicals without proper training or supervision. Accidental spills can cause burns or damage surfaces.
Summarizing the HCl + NaOH Reaction
- Reaction Type: Acid-base neutralization.
- Products: Water (H₂O) and sodium chloride (NaCl).
- Applications: Chemical manufacturing, wastewater treatment, and household cleaning.
Checklist for Safe Handling
- Wear protective gear (gloves, goggles).
- Ensure proper ventilation.
- Measure chemicals accurately.
- Dispose of waste safely.
Understanding the HCl + NaOH reaction is crucial for anyone involved in chemistry, whether for academic purposes or practical applications. By grasping the fundamentals of this neutralization process, you can appreciate its significance in both scientific and industrial contexts.
What is the product of the HCl + NaOH reaction?
+
The reaction produces water (H₂O) and sodium chloride (NaCl).
Is the HCl + NaOH reaction exothermic or endothermic?
+
The reaction is exothermic, meaning it releases heat.
What safety precautions should be taken when handling HCl and NaOH?
+
Always wear protective gear, ensure proper ventilation, and handle chemicals with care to avoid burns or spills.