Understanding the IMF of CO2: A Comprehensive Guide

Understanding the IMF of CO2 is crucial for anyone looking to grasp the intricacies of carbon dioxide’s molecular structure and its implications on climate change. The Intermolecular Forces (IMF) of CO2 play a significant role in its behavior, from its role in the atmosphere to industrial applications. Whether you’re a student, researcher, or environmentally conscious individual, this guide breaks down the concept into digestible insights. We’ll explore the types of IMF in CO2, their impact, and why it matters in today’s world. Let’s dive in! (Intermolecular Forces, CO2 Structure, Climate Change)
What Are Intermolecular Forces (IMF)?

Intermolecular Forces (IMF) are the attractions between molecules that dictate their physical properties, such as boiling point, melting point, and solubility. Unlike covalent bonds, which hold atoms together within a molecule, IMF act between molecules. Understanding IMF is key to analyzing how substances like CO2 behave in different conditions. (Intermolecular Forces, Molecular Interactions)
Types of IMF in CO2

CO2 primarily exhibits London Dispersion Forces (LDF), a type of IMF arising from temporary uneven electron distributions. Despite its linear, nonpolar structure, CO2 molecules can experience weak attractions due to LDF. Here’s a breakdown:
- London Dispersion Forces (LDF): Weak forces present in all molecules, including CO2.
- No Dipole-Dipole Forces: CO2’s linear symmetry cancels out any permanent dipoles.
- No Hydrogen Bonding: CO2 lacks hydrogen atoms bonded to highly electronegative elements.
📌 Note: While CO2’s IMF are weak, they still influence its phase changes and interactions with other substances. (London Dispersion Forces, CO2 Polarity)
Why IMF in CO2 Matters

The IMF of CO2 have far-reaching implications, especially in the context of climate change and industrial processes. Here’s why it’s important:
- Atmospheric Behavior: Weak IMF allow CO2 to remain gaseous at room temperature, contributing to its role as a greenhouse gas.
- Industrial Applications: Understanding IMF helps optimize CO2 capture and storage technologies.
- Environmental Impact: CO2’s IMF influence its solubility in oceans, affecting marine ecosystems. (Greenhouse Gas, CO2 Capture)
Comparing IMF in CO2 and Other Molecules

To better understand CO2’s IMF, let’s compare it with other molecules:
| Molecule | IMF Type | Polarity |
|---|---|---|
| CO2 | London Dispersion Forces | Nonpolar |
| H2O | Hydrogen Bonding | Polar |
| CH4 | London Dispersion Forces | Nonpolar |
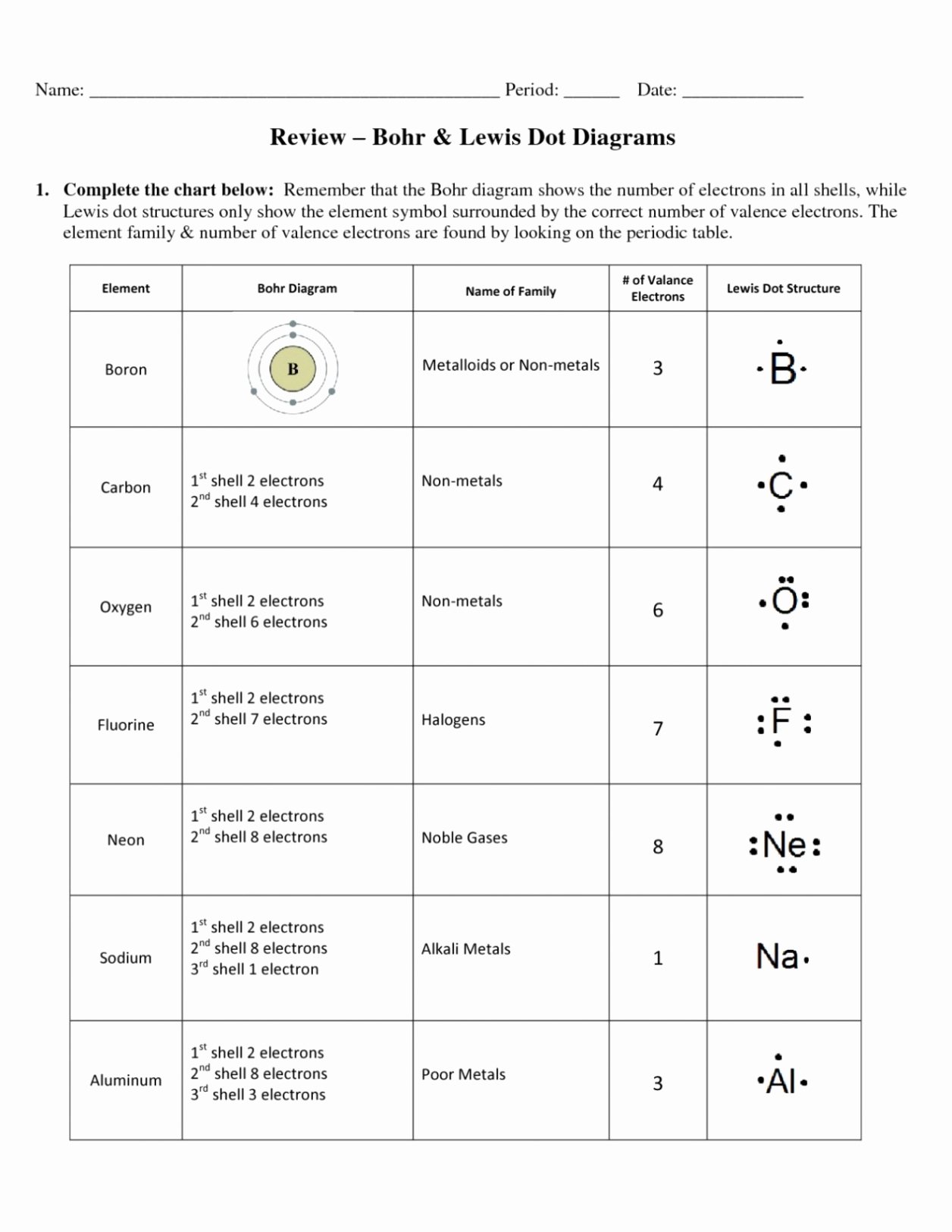
This comparison highlights how CO2’s nonpolar nature and weak IMF differentiate it from polar molecules like H2O. (Molecular Comparisons, Polarity)
Practical Applications of IMF in CO2
The understanding of CO2’s IMF has led to innovative solutions in various fields:
- Carbon Capture Technology: Engineers design materials that exploit CO2’s weak IMF for efficient capture.
- Sustainable Energy: CO2’s IMF are studied to improve its conversion into usable fuels.
- Environmental Monitoring: Knowledge of IMF helps predict CO2’s behavior in the atmosphere and oceans. (Carbon Capture, Sustainable Energy)
Checklist: Key Takeaways on IMF of CO2
- CO2 exhibits only London Dispersion Forces due to its nonpolar, linear structure.
- Weak IMF in CO2 influence its gaseous state and role in climate change.
- Understanding IMF is vital for developing technologies like carbon capture and sustainable energy solutions.
The IMF of CO2 may seem like a small detail, but its impact on science, industry, and the environment is profound. By understanding these forces, we can better address challenges like climate change and innovate for a sustainable future. Whether you’re studying chemistry or exploring green technologies, this knowledge is a valuable tool. (Intermolecular Forces, Climate Solutions)
What are the main types of IMF in CO2?
+
CO2 primarily exhibits London Dispersion Forces (LDF), as it is a nonpolar molecule with no dipole-dipole forces or hydrogen bonding. (London Dispersion Forces)
How do IMF in CO2 affect climate change?
+
Weak IMF allow CO2 to remain gaseous, contributing to its role as a greenhouse gas and trapping heat in the atmosphere. (Greenhouse Gas)
Why is CO2 nonpolar despite having polar bonds?
+
CO2’s linear structure causes the polar bonds to cancel each other out, resulting in a nonpolar molecule. (CO2 Polarity)


