Understanding Intermolecular Forces in Methane (CH4)

Methane (CH₄) is one of the simplest organic compounds, yet its intermolecular forces play a crucial role in its physical and chemical properties. Understanding these forces is essential for applications in chemistry, energy, and environmental science. In this post, we’ll explore the intermolecular forces in methane, their impact, and why they matter. Whether you’re a student, researcher, or industry professional, this guide will provide valuable insights into methane’s molecular interactions, van der Waals forces, and dipole-induced dipole forces.
What Are Intermolecular Forces in Methane?

Methane is a nonpolar molecule with a symmetrical tetrahedral structure. The primary intermolecular forces in methane are London dispersion forces (a type of van der Waals force). These forces arise due to temporary fluctuations in electron distribution, creating instantaneous dipoles that induce dipoles in neighboring molecules.
📌 Note: Unlike polar molecules, methane does not exhibit dipole-dipole interactions or hydrogen bonding.
Why London Dispersion Forces Dominate in Methane

London dispersion forces are the weakest intermolecular forces but are significant in nonpolar molecules like methane. Here’s why they dominate:
- Symmetrical Structure: Methane’s tetrahedral shape ensures even electron distribution, preventing permanent dipoles.
- Small Size: As a small molecule, methane’s electrons are closer to the nucleus, reducing the strength of temporary dipoles.
- Nonpolarity: The C-H bonds are nonpolar, eliminating dipole-dipole interactions.
Impact of Intermolecular Forces on Methane’s Properties

The intermolecular forces in methane directly influence its physical properties:
| Property | Explanation |
|---|---|
| Boiling Point | Low boiling point (-161.5°C) due to weak London dispersion forces. |
| Solubility | Insoluble in water but soluble in nonpolar solvents like benzene. |
| Volatility | Highly volatile due to weak intermolecular forces. |
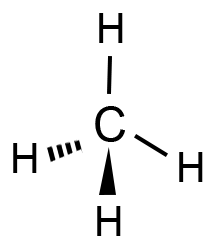
Methane in Industrial and Environmental Contexts

Understanding methane’s intermolecular forces is crucial for:
- Energy Production: Methane is a primary component of natural gas, and its volatility affects storage and transportation.
- Climate Change: Methane is a potent greenhouse gas, and its intermolecular forces influence its behavior in the atmosphere.
- Chemical Synthesis: Methane’s reactivity in industrial processes depends on its molecular interactions.
Key Takeaways: Checklist for Understanding Methane’s Intermolecular Forces
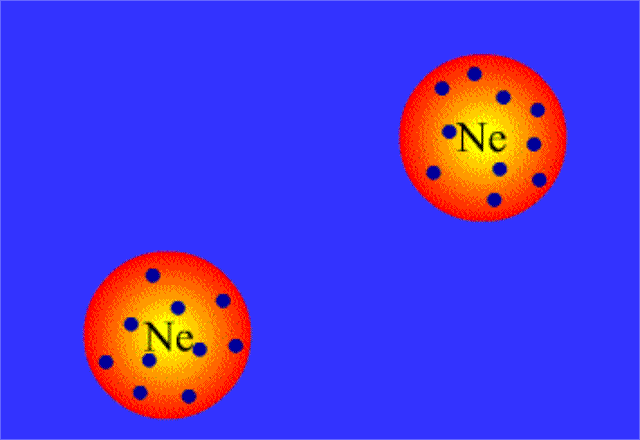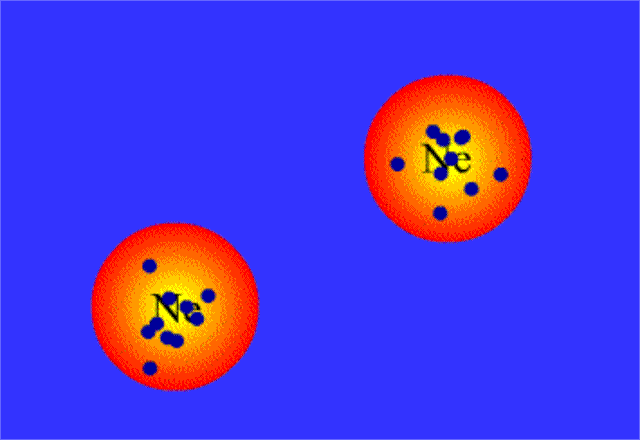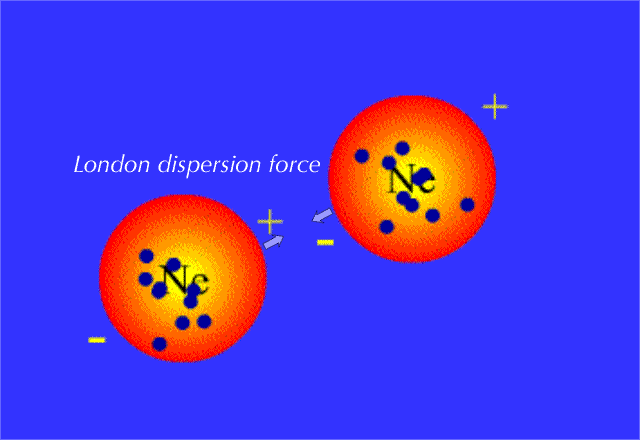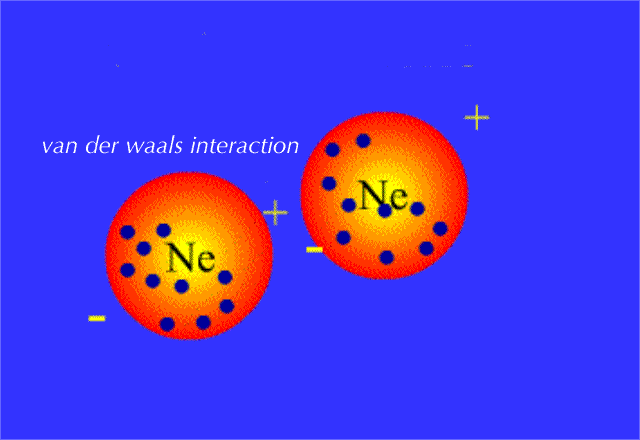
- Identify the Dominant Force: London dispersion forces are the primary intermolecular forces in methane.
- Relate to Properties: Connect weak forces to low boiling point, volatility, and solubility.
- Apply to Real-World Scenarios: Consider methane’s role in energy, climate, and chemistry.
What are the intermolecular forces in methane?
+Methane’s primary intermolecular force is London dispersion forces, a type of van der Waals force.
Why doesn’t methane exhibit dipole-dipole interactions?
+Methane is nonpolar due to its symmetrical tetrahedral structure and nonpolar C-H bonds.
How do intermolecular forces affect methane’s boiling point?
+Weak London dispersion forces result in a low boiling point of -161.5°C.
In summary, intermolecular forces in methane are primarily London dispersion forces, which dictate its physical properties and applications. By understanding these forces, we can better grasp methane’s role in science and industry. Whether you’re studying chemistry or working in energy, this knowledge is invaluable. (intermolecular forces in methane, methane molecular structure, London dispersion forces, van der Waals forces)



