Is KOH a Strong or Weak Base?
Opening Paragraph:
Potassium hydroxide (KOH) is a widely used chemical compound in various industries, from soap manufacturing to batteries. One common question that arises is: Is KOH a strong or weak base? Understanding its properties is crucial for both academic and practical applications. In this post, we’ll explore the characteristics of KOH, its behavior in solutions, and how it compares to other bases. Whether you’re a student, researcher, or industry professional, this guide will provide clarity on KOH’s strength as a base. (strong base, weak base, chemical properties)
What is Potassium Hydroxide (KOH)?
Potassium hydroxide, also known as caustic potash, is an inorganic compound with the chemical formula KOH. It is a white solid that is highly soluble in water and forms strongly alkaline solutions. KOH is commonly used in chemical synthesis, pH regulation, and as a key ingredient in many industrial processes.
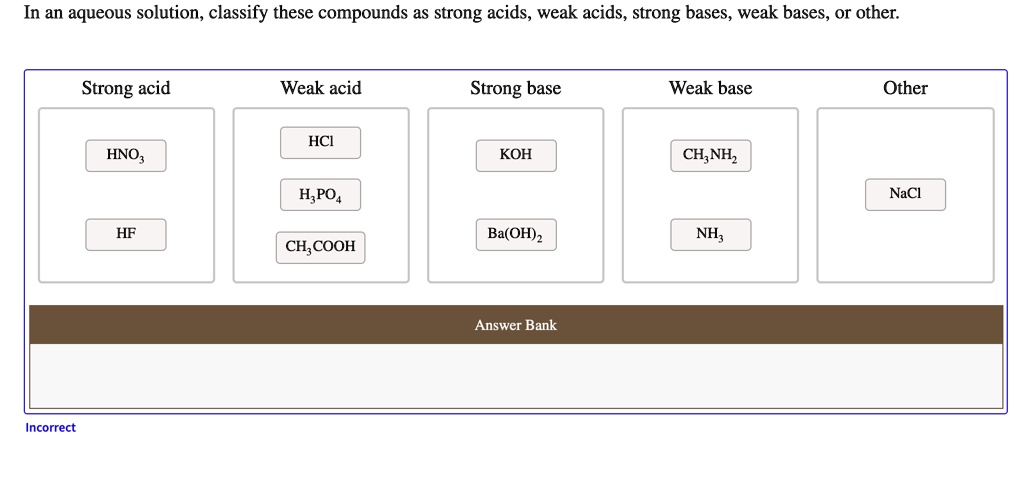
Is KOH a Strong Base?

Yes, KOH is a strong base. A strong base is one that dissociates completely in water, releasing a high concentration of hydroxide ions (OH⁻). When KOH dissolves in water, it breaks down entirely into potassium ions (K⁺) and hydroxide ions (OH⁻), as shown in the equation:
KOH → K⁺ + OH⁻
This complete dissociation results in a high pH level, typically above 12, making KOH a powerful alkaline substance.
| Property | KOH (Strong Base) | Weak Base |
|---|---|---|
| Dissociation in Water | Complete | Partial |
| pH Level | High (above 12) | Moderate (around 7-11) |
| Conductivity | High | Low to Moderate |

How Does KOH Compare to Other Bases?

KOH is often compared to sodium hydroxide (NaOH), another strong base. Both are highly soluble and dissociate completely in water, but KOH is slightly more soluble in alcohol-based solutions. Weak bases, such as ammonia (NH₃), only partially dissociate, resulting in lower pH levels and weaker alkaline properties.
Practical Applications of KOH as a Strong Base

KOH’s strength as a base makes it ideal for:
- Soap and biodiesel production: It catalyzes the saponification process.
- Battery manufacturing: Used in alkaline batteries.
- Chemical synthesis: Acts as a reagent in various reactions.
- pH adjustment: Helps neutralize acidic solutions in industrial processes.
💡 Note: Always handle KOH with care, as it can cause skin burns and eye damage due to its strong alkaline nature.
Key Takeaways

- KOH is a strong base due to its complete dissociation in water.
- It has high solubility and conductivity, making it versatile for industrial use.
- Always prioritize safety when working with KOH.
Final Thoughts:
Understanding whether KOH is a strong or weak base is essential for its effective use in various applications. Its strong alkaline properties make it a valuable chemical compound, but proper handling is crucial to avoid hazards. (chemical compounds, industrial applications, safety precautions)
What makes KOH a strong base?
+
KOH is a strong base because it dissociates completely in water, releasing a high concentration of hydroxide ions (OH⁻).
How does KOH differ from NaOH?
+
Both KOH and NaOH are strong bases, but KOH is slightly more soluble in alcohol-based solutions compared to NaOH.
Is KOH safe to use?
+
KOH is highly corrosive and can cause skin burns and eye damage. Always wear protective gear when handling it.