Lewis Dot Diagram for Fluorine Explained Simply Mastering Fluorine's Lewis Dot Structure Easily How to Draw Fluorine’s Lewis Dot Diagram Fluorine Lewis Dot Diagram: Quick Guide Understanding Fluorine’s Lewis Structure Basics
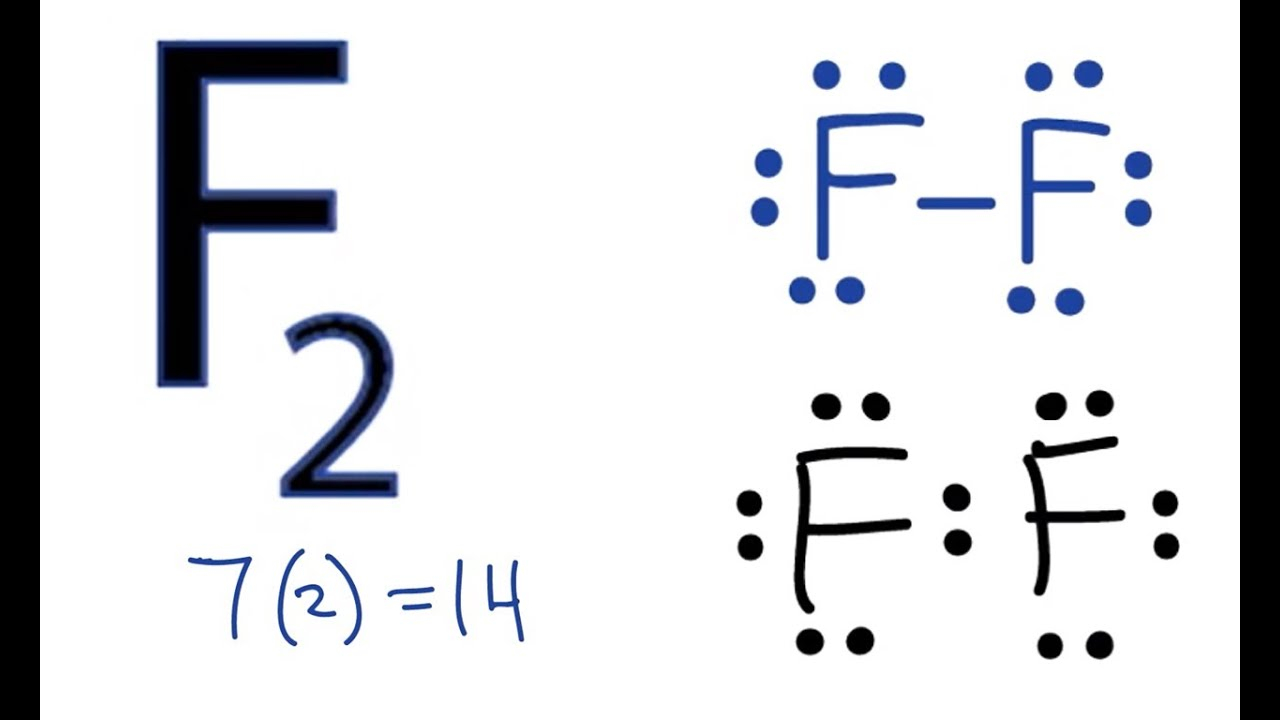
Understanding the Lewis Dot Diagram for Fluorine is essential for anyone studying chemistry. Fluorine, a highly reactive halogen, has a simple yet crucial Lewis structure that reflects its electron configuration. Whether you're a student, educator, or chemistry enthusiast, mastering this concept will help you grasp more complex chemical principles. In this guide, we’ll break down how to draw Fluorine’s Lewis Dot Diagram step-by-step, ensuring you can confidently tackle similar problems in the future. Let’s dive in! (Lewis Dot Diagram for Fluorine Explained Simply, Mastering Fluorine's Lewis Dot Structure Easily)
What is a Lewis Dot Diagram?

A Lewis Dot Diagram, also known as an electron dot diagram, visually represents the valence electrons of an atom. It uses dots around the chemical symbol to show how electrons are arranged. For Fluorine’s Lewis Dot Diagram, this tool helps illustrate its electron distribution, which is vital for understanding its bonding behavior. (Understanding Fluorine’s Lewis Structure Basics)
How to Draw Fluorine’s Lewis Dot Diagram

Fluorine (F) has an atomic number of 9, meaning it has 9 electrons. However, only the valence electrons (electrons in the outermost shell) are shown in the Lewis diagram. Here’s a simple step-by-step process:
- Identify the symbol: Write the symbol “F” for Fluorine.
- Determine valence electrons: Fluorine has 7 valence electrons.
- Place dots around the symbol: Arrange the 7 dots in pairs around the symbol, starting from the top and moving clockwise.
📌 Note: Fluorine’s Lewis Dot Diagram will have 7 dots, with each side of the symbol having one pair and one single dot. (Fluorine Lewis Dot Diagram: Quick Guide)
Why Fluorine’s Lewis Structure is Important

Fluorine’s Lewis Dot Diagram is crucial for understanding its chemical properties. As the most electronegative element, Fluorine often forms strong bonds with other elements. Its Lewis structure helps predict how it interacts in compounds like Hydrofluoric Acid (HF) or Fluorine Gas (F₂). (Mastering Fluorine’s Lewis Dot Structure Easily)
Checklist: Drawing Fluorine’s Lewis Dot Diagram
- Write the symbol “F” for Fluorine.
- Identify 7 valence electrons.
- Place dots in pairs around the symbol.
- Ensure one pair and one single dot on each side.
In summary, Fluorine’s Lewis Dot Diagram is a straightforward representation of its 7 valence electrons. By following the steps outlined above, you can easily draw and understand this structure. This knowledge is foundational for exploring more advanced chemistry topics, such as molecular bonding and reactivity. (Lewis Dot Diagram for Fluorine Explained Simply, How to Draw Fluorine’s Lewis Dot Diagram)
What are valence electrons?
+
Valence electrons are the electrons in the outermost shell of an atom, involved in chemical bonding.
Why does Fluorine have 7 dots in its Lewis diagram?
+
Fluorine has 7 valence electrons, which are represented as dots in its Lewis Dot Diagram.
How does Fluorine’s Lewis structure relate to its reactivity?
+
Fluorine’s 7 valence electrons make it highly reactive, as it seeks to gain one more electron to complete its octet.



