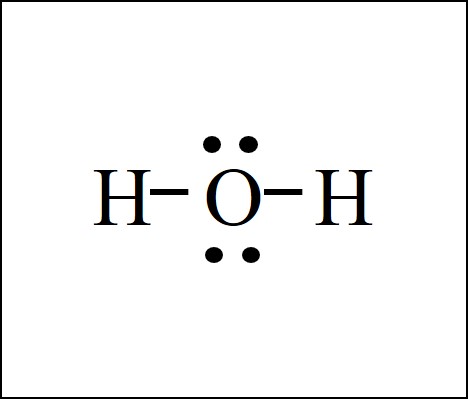
lewis dot structure h20
<!DOCTYPE html>
Understanding the Lewis dot structure of H2O is essential for grasping the molecular geometry and bonding in water molecules. This simple yet powerful representation helps visualize how atoms share electrons to form stable molecules. Whether you’re a student, educator, or chemistry enthusiast, mastering this concept is crucial for further studies in chemistry.
What is a Lewis Dot Structure?
A Lewis dot structure is a diagram that shows the bonding between atoms in a molecule and the lone pairs of electrons that may exist. It uses dots to represent valence electrons and lines to depict chemical bonds. For H2O, the Lewis structure helps us understand how oxygen and hydrogen atoms bond to form water.
Steps to Draw the Lewis Dot Structure of H2O

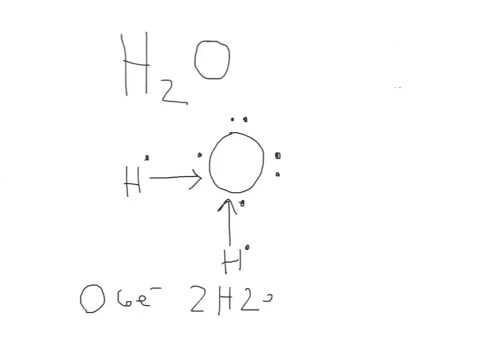
Drawing the Lewis dot structure of H2O involves a few straightforward steps:
- Step 1: Determine the total number of valence electrons. Oxygen has 6 valence electrons, and each hydrogen has 1, totaling 8 electrons.
- Step 2: Place the atoms. Oxygen is the central atom, with two hydrogen atoms bonded to it.
- Step 3: Connect the atoms with single bonds. This uses up 4 electrons, leaving 4 for lone pairs on oxygen.
- Step 4: Add lone pairs. Place the remaining electrons as lone pairs on the oxygen atom to satisfy the octet rule.
Key Features of H2O Lewis Structure

The Lewis dot structure of H2O highlights several important aspects:
- Central Atom: Oxygen acts as the central atom, forming bonds with two hydrogen atoms.
- Bonding Pairs: Two bonding pairs (O-H bonds) are present.
- Lone Pairs: Two lone pairs of electrons reside on the oxygen atom.
| Atom | Valence Electrons | Role in H2O |
|---|---|---|
| Oxygen | 6 | Central atom with lone pairs |
| Hydrogen | 1 | Bonded to oxygen |

📌 Note: Ensure the octet rule is satisfied for oxygen, while hydrogen has a duet of electrons.
Importance of H2O Lewis Structure in Chemistry

The Lewis dot structure of H2O is vital for understanding its properties, such as polarity and hydrogen bonding. It also serves as a foundation for predicting molecular geometry and reactivity in chemical reactions.
Checklist for Drawing Lewis Structures
- Count total valence electrons.
- Identify the central atom.
- Form single bonds between atoms.
- Add lone pairs to satisfy the octet rule.
- Check for stability and minimize formal charges.
Mastering the Lewis dot structure of H2O opens doors to understanding more complex molecules and their behaviors. Whether for academic purposes or practical applications, this knowledge is invaluable in the field of chemistry,lewis dot structure,molecular geometry,chemical bonding.
What is the Lewis dot structure of H2O?
+The Lewis dot structure of H2O shows oxygen as the central atom with two hydrogen atoms bonded to it. Oxygen has two lone pairs of electrons.
How many valence electrons does H2O have?
+H2O has a total of 8 valence electrons: 6 from oxygen and 1 from each hydrogen atom.
Why is the Lewis structure of H2O important?
+It helps understand the molecular geometry, polarity, and chemical properties of water, which are fundamental in chemistry.


