lewis dot structure of hi
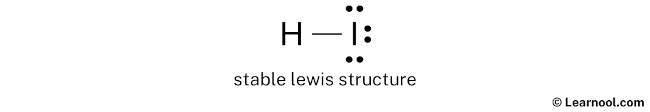
Understanding the Lewis Dot Structure of HI (Hydrogen Iodide)
Hydrogen iodide (HI) is a simple diatomic molecule composed of hydrogen (H) and iodine (I). Its Lewis dot structure is a fundamental concept in chemistry, helping us visualize the arrangement of electrons and bonds. This structure is crucial for understanding HI’s chemical properties and reactivity. Whether you’re a student, educator, or chemistry enthusiast, mastering the Lewis dot structure of HI is essential. (Lewis dot structure, HI molecule, chemical bonding)
What is a Lewis Dot Structure?
A Lewis dot structure represents the distribution of valence electrons in an atom or molecule. It uses dots to symbolize electrons and lines to indicate chemical bonds. For HI, this structure illustrates how hydrogen and iodine share electrons to form a stable molecule.
Steps to Draw the Lewis Dot Structure of HI
Determine the Total Valence Electrons
- Hydrogen (H) has 1 valence electron.
- Iodine (I) has 7 valence electrons.
- Total: 1 + 7 = 8 valence electrons.
- Hydrogen (H) has 1 valence electron.
Identify the Central Atom
- In HI, iodine (I) is the central atom due to its higher electronegativity.
- In HI, iodine (I) is the central atom due to its higher electronegativity.
Form Bonds and Distribute Electrons
- Place H and I next to each other and connect them with a single bond, representing 2 shared electrons.
- Distribute the remaining 6 electrons around iodine as lone pairs.
- Place H and I next to each other and connect them with a single bond, representing 2 shared electrons.
📌 Note: Hydrogen can only form one bond due to its single valence electron.
Lewis Dot Structure of HI: Visual Representation
The Lewis dot structure of HI can be represented as follows:
H:I:
- H is connected to I by a single bond.
- Iodine has 3 pairs of lone electrons (6 dots) around it.
Key Properties of HI Based on Its Lewis Structure
- Polarity: HI is a polar molecule due to the electronegativity difference between H and I.
- Reactivity: The structure explains HI’s acidic nature and its role in chemical reactions.
Checklist for Drawing Lewis Dot Structures
- Count total valence electrons.
- Identify the central atom.
- Form bonds and distribute remaining electrons.
- Ensure all atoms satisfy the octet rule (except hydrogen).
To summarize, the Lewis dot structure of HI is a straightforward yet powerful tool for understanding its molecular composition and behavior. By following the steps outlined above, you can easily draw and interpret this structure. Whether for academic purposes or practical applications, mastering this concept is invaluable. (Lewis dot structure, HI molecule, chemical bonding)
What is the Lewis dot structure of HI?
+
The Lewis dot structure of HI shows hydrogen (H) connected to iodine (I) by a single bond, with iodine having 3 pairs of lone electrons.
Why is iodine the central atom in HI?
+
Iodine is the central atom due to its higher electronegativity and ability to hold more electrons compared to hydrogen.
Is HI a polar molecule?
+
Yes, HI is polar due to the significant electronegativity difference between hydrogen and iodine.



