lewis structure cacl2

Understanding the Lewis structure of CaCl₂ is essential for grasping its chemical properties and bonding behavior. Calcium chloride (CaCl₂) is an ionic compound widely used in industries, medicine, and even as a desiccant. By learning its Lewis structure, you can better comprehend its electron distribution and molecular geometry. This guide breaks down the process step-by-step, ensuring clarity for both students and professionals. Whether you're studying chemistry or exploring its applications, this post provides valuable insights into CaCl₂'s structure and significance, (lewis structure, chemical bonding, ionic compounds)
What is the Lewis Structure of CaCl₂?

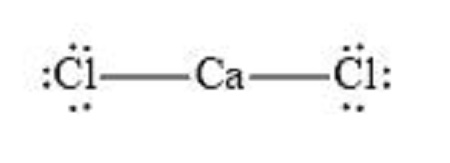
The Lewis structure of CaCl₂ represents the arrangement of electrons in this ionic compound. Unlike covalent compounds, CaCl₂ consists of calcium (Ca²⁺) and chloride (Cl⁻) ions held together by electrostatic forces. Calcium donates two electrons to form Ca²⁺, while each chlorine atom gains one electron to form Cl⁻. This electron transfer results in a stable, neutral compound. The Lewis structure simplifies this by focusing on the electron distribution around the ions, (electron configuration, ionic bonding, calcium chloride)
Step-by-Step Guide to Drawing the Lewis Structure of CaCl₂

Step 1: Determine the Total Number of Valence Electrons
Calcium (Ca) has 2 valence electrons, and each chlorine (Cl) atom has 7 valence electrons. Since CaCl₂ has two chlorine atoms, the total valence electrons are: 2 (Ca) + 2 × 7 (Cl) = 16 electrons. This step is crucial for understanding the electron distribution, (valence electrons, electron counting, molecular structure)
Step 2: Identify the Central Atom
In CaCl₂, calcium (Ca) acts as the central atom. However, since it’s an ionic compound, the structure is represented by separate ions rather than a central atom with bonded atoms. This distinction is key to visualizing CaCl₂’s structure, (central atom, ionic structure, chemical formula)
Step 3: Distribute Electrons to Form Ions
Calcium loses its 2 valence electrons to form Ca²⁺, while each chlorine gains one electron to form Cl⁻. The Lewis structure shows Ca²⁺ surrounded by two Cl⁻ ions, indicating the ionic bond. This distribution ensures a stable electron configuration, (electron distribution, ion formation, stable compounds)
💡 Note: CaCl₂ does not have a traditional Lewis dot structure because it is an ionic compound, not a covalent molecule.
Key Properties of CaCl₂ Based on Its Lewis Structure

The Lewis structure of CaCl₂ highlights its ionic nature, which explains its properties: - High Melting Point: Strong ionic bonds require significant energy to break. - Solubility in Water: CaCl₂ dissociates into Ca²⁺ and Cl⁻ ions in water. - Electrical Conductivity: In molten or aqueous states, it conducts electricity due to free-moving ions. These properties make CaCl₂ valuable in de-icing, water treatment, and medical applications, (chemical properties, solubility, conductivity)
Practical Applications of CaCl₂
CaCl₂’s unique structure and properties lend it to various uses: - De-icing Roads: Its ability to lower freezing points makes it effective in cold climates. - Food Additive: Used as a firming agent in canned vegetables and pickles. - Medicine: Administered to treat calcium deficiencies or magnesium toxicity. Understanding its Lewis structure enhances its application in these fields, (industrial uses, medical applications, food additives)
Checklist for Drawing the Lewis Structure of CaCl₂
- Count the total valence electrons (16 for CaCl₂)
- Identify calcium as the central ion
- Form Ca²⁺ and Cl⁻ ions by transferring electrons
- Represent the ionic bond between Ca²⁺ and Cl⁻ ions
Mastering the Lewis structure of CaCl₂ provides a foundation for understanding its ionic nature and practical applications. By following the steps outlined, you can confidently analyze its electron distribution and properties. Whether for academic purposes or industrial use, this knowledge is invaluable. Explore related topics like chemical bonding or ionic compounds to deepen your understanding, (lewis structure, chemical bonding, ionic compounds)
Why doesn’t CaCl₂ have a traditional Lewis structure?
+
CaCl₂ is an ionic compound, not a covalent molecule, so it’s represented by separate ions (Ca²⁺ and Cl⁻) rather than shared electrons.
How does the Lewis structure of CaCl₂ explain its solubility in water?
+
The ionic nature of CaCl₂ allows it to dissociate into Ca²⁺ and Cl⁻ ions in water, making it highly soluble.
What is the role of calcium in the Lewis structure of CaCl₂?
+
Calcium donates its 2 valence electrons to form Ca²⁺, which then attracts two Cl⁻ ions to form the compound.



