Mastering Lewis Structure for Hydrogen Molecules How to Draw Hydrogen Lewis Structures Easily Understanding Hydrogen in Lewis Dot Structures Simple Guide to Hydrogen Lewis Structure Drawing Hydrogen Lewis Structure Explained in Minutes
<!DOCTYPE html>
Understanding how to draw Lewis structures is essential in chemistry, especially for simple molecules like hydrogen. Whether you’re a student or a professional, mastering Hydrogen Lewis Structures can significantly enhance your grasp of molecular bonding. This guide will walk you through the process step-by-step, ensuring you can draw these structures with ease. (Lewis Structure for Hydrogen Molecules, Hydrogen Lewis Dot Structures)
Understanding Hydrogen in Lewis Dot Structures

Hydrogen is the simplest element, with only one electron in its outermost shell. In Lewis Dot Structures, hydrogen is represented by the symbol H with one dot, symbolizing its single valence electron. This simplicity makes hydrogen an ideal starting point for learning how to draw Lewis structures. (Hydrogen Lewis Structure Explained, Lewis Structure Drawing)
How to Draw Hydrogen Lewis Structures Easily

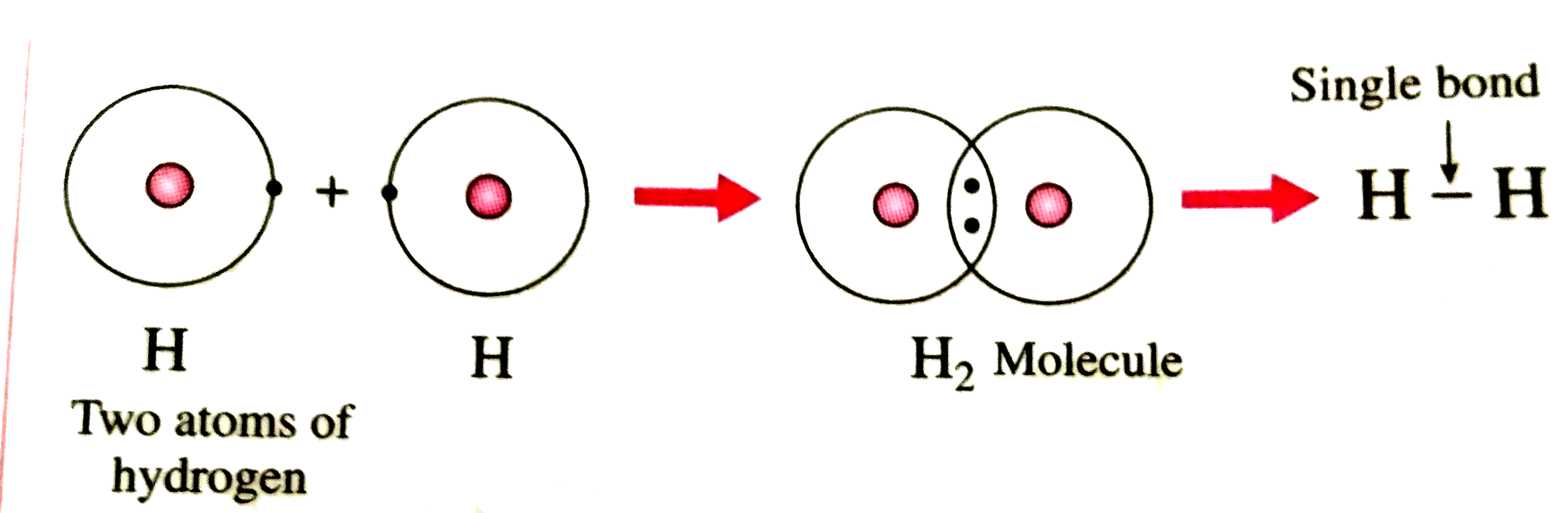
Drawing the Lewis structure for a hydrogen molecule (H₂) involves a few straightforward steps:
- Step 1: Count the Total Valence Electrons - Hydrogen has 1 valence electron per atom, so H₂ has 2 valence electrons in total. (Simple Guide to Hydrogen Lewis Structure)
- Step 2: Arrange the Atoms - Place the two hydrogen atoms next to each other.
- Step 3: Form Bonds - Connect the hydrogen atoms with a single covalent bond, using 2 electrons.
- Step 4: Check Stability - Each hydrogen atom now has a full outer shell (duet rule), making the structure stable.
💡 Note: Hydrogen only needs 2 electrons to achieve a stable configuration, unlike most other elements that require 8.
Simple Guide to Hydrogen Lewis Structure Drawing

For more complex hydrogen-containing molecules, follow these additional tips:
- Identify the Central Atom - If hydrogen is bonded to another element, that element is usually the central atom.
- Distribute Electrons - Place electrons around atoms to satisfy the octet rule (or duet rule for hydrogen).
- Check Formal Charges - Ensure the structure has the lowest possible formal charges for stability.
| Step | Description |
|---|---|
| 1 | Count valence electrons |
| 2 | Arrange atoms |
| 3 | Form bonds |
| 4 | Check stability |

Hydrogen Lewis Structure Explained in Minutes

In summary, drawing the Lewis structure for hydrogen molecules is a quick and straightforward process. By following these steps, you’ll be able to represent hydrogen’s bonding accurately in no time. (Mastering Lewis Structure for Hydrogen Molecules, Hydrogen in Lewis Dot Structures)
✨ Note: Practice with different hydrogen-containing molecules to reinforce your skills.
Mastering Hydrogen Lewis Structures is a fundamental skill in chemistry. With this guide, you’ll be able to draw these structures confidently, whether for academic purposes or professional applications. Keep practicing, and soon you’ll be an expert in Lewis Structure Drawing! (Hydrogen Lewis Structure Explained, Simple Guide to Hydrogen Lewis Structure)
What is the Lewis structure for a hydrogen molecule (H₂)?
+The Lewis structure for H₂ consists of two hydrogen atoms connected by a single covalent bond, with each hydrogen having no lone pairs.
Why does hydrogen only need 2 electrons in its Lewis structure?
+Hydrogen follows the duet rule, meaning it only needs 2 electrons to achieve a stable electron configuration.
Can hydrogen form double or triple bonds in Lewis structures?
+No, hydrogen typically forms only single bonds due to its single valence electron.


