lewis structure of c4h10

Understanding the Lewis structure of C4H10 is essential for anyone studying organic chemistry or working with hydrocarbons. C4H10, also known as butane, is a simple yet important molecule with two structural isomers: n-butane and isobutane. This post will guide you through drawing the Lewis structure, explain its significance, and provide practical insights for both informational and commercial purposes. (lewis structure of c4h10, butane structure, organic chemistry basics)
What is the Lewis Structure of C4H10?

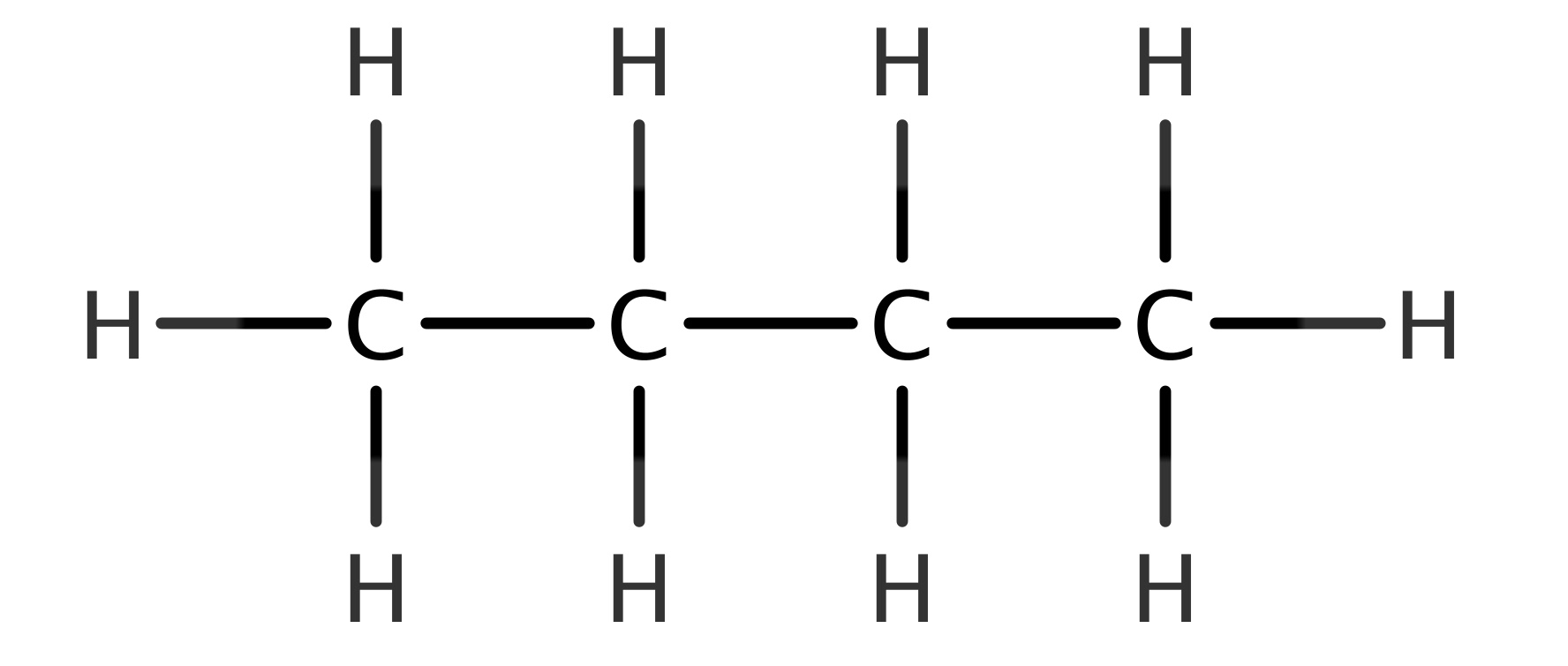
The Lewis structure of a molecule represents the arrangement of atoms and valence electrons using dots and lines. For C4H10, the structure helps visualize how carbon and hydrogen atoms bond to form butane. Here’s a breakdown:
Steps to Draw the Lewis Structure of C4H10
- Step 1: Determine Total Valence Electrons – Carbon has 4 valence electrons, and hydrogen has 1. For C4H10, calculate: (4 × 4) + (1 × 10) = 18 electrons.
- Step 2: Identify the Central Atom – In n-butane, carbon atoms form a straight chain, while isobutane has a branched structure.
- Step 3: Connect Atoms with Single Bonds – Use lines to represent bonds between carbon and hydrogen atoms.
- Step 4: Distribute Remaining Electrons – Place remaining electrons as lone pairs, ensuring all atoms satisfy the octet rule.
✨ Note: Hydrogen atoms always form single bonds and never have lone pairs.
Types of C4H10 Isomers: N-Butane vs. Isobutane
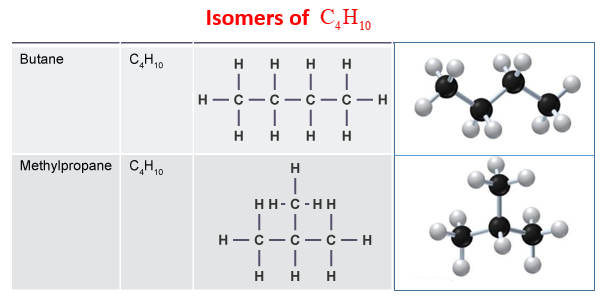
C4H10 exists as two isomers: n-butane and isobutane. Their Lewis structures differ in carbon atom arrangement:
| Isomer | Structure | Key Feature |
|---|---|---|
| N-Butane | Straight chain of 4 carbon atoms | Linear arrangement |
| Isobutane | Branched chain with 3 carbon atoms and 1 methyl group | Compact structure |

Why Isomer Structures Matter
Understanding isomers is crucial for applications in petrochemical industries, where butane is used as a fuel or refrigerant. Isomers affect physical properties like boiling point and reactivity. (butane isomers, petrochemical applications, organic compounds)
Practical Applications of C4H10
Butane is widely used in commercial products. Here’s how its Lewis structure relates to its applications:
- Fuel Source – Butane’s stable structure makes it ideal for lighters and portable stoves.
- Refrigerant – Isobutane is used in eco-friendly refrigeration systems.
- Chemical Feedstock – Butane is a precursor in producing plastics and synthetic rubber.
Mastering the Lewis structure of C4H10 provides a foundation for understanding its properties and applications. Whether you’re a student or a professional, this knowledge is invaluable in chemistry and industry. (lewis structure of c4h10, butane applications, chemistry fundamentals)
What is the difference between n-butane and isobutane?
+N-butane has a straight chain of 4 carbon atoms, while isobutane has a branched structure with 3 carbon atoms and a methyl group.
How many valence electrons does C4H10 have?
+C4H10 has 18 valence electrons: (4 × 4) for carbon and (1 × 10) for hydrogen.
Why is butane used as a refrigerant?
+Isobutane is eco-friendly and efficient, making it a preferred choice for refrigeration systems.



