Molar Mass of C7H6O3: Quick Calculation Guide

Understanding the molar mass of C7H6O3 is essential for various applications in chemistry, including stoichiometry, chemical reactions, and laboratory experiments. This compound, known as benzoic acid or methylbenzoate, is widely used in industries such as food preservation, pharmaceuticals, and fragrances. Calculating its molar mass is straightforward once you know the atomic masses of carbon ©, hydrogen (H), and oxygen (O). Let’s dive into the step-by-step process to determine the molar mass of C7H6O3 efficiently.
What is Molar Mass and Why is it Important?
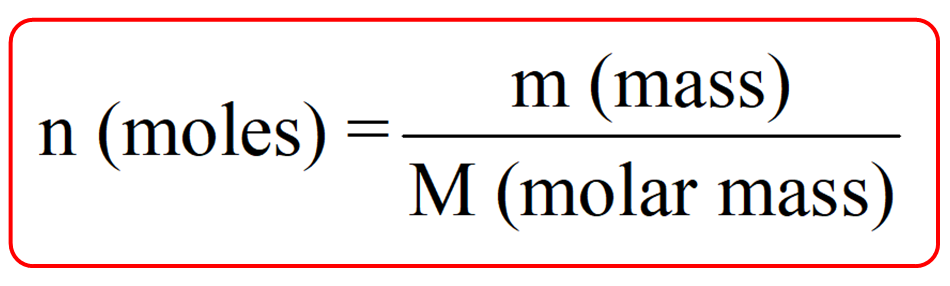
Molar mass represents the mass of one mole of a substance and is expressed in grams per mole (g/mol). It is a fundamental concept in chemistry, crucial for balancing chemical equations, determining reaction yields, and understanding molecular structures. For C7H6O3, calculating its molar mass helps in precise measurements and accurate experimental results.
Step-by-Step Calculation of the Molar Mass of C7H6O3

To calculate the molar mass of C7H6O3, follow these simple steps:
Identify the Atomic Masses
- Carbon ©: 12.01 g/mol
- Hydrogen (H): 1.01 g/mol
- Oxygen (O): 16.00 g/mol
- Carbon ©: 12.01 g/mol
Count the Atoms in the Formula
- Carbon ©: 7 atoms
- Hydrogen (H): 6 atoms
- Oxygen (O): 3 atoms
- Carbon ©: 7 atoms
Multiply Atomic Masses by Atom Counts
- Carbon: 7 × 12.01 = 84.07 g/mol
- Hydrogen: 6 × 1.01 = 6.06 g/mol
- Oxygen: 3 × 16.00 = 48.00 g/mol
- Carbon: 7 × 12.01 = 84.07 g/mol
Sum the Results
Total molar mass = 84.07 + 6.06 + 48.00 = 138.13 g/mol
📌 Note: Always double-check atomic masses and calculations to ensure accuracy.
Practical Applications of Knowing the Molar Mass of C7H6O3

Understanding the molar mass of C7H6O3 is beneficial in various fields:
- Pharmaceuticals: Formulating medications with precise concentrations.
- Food Industry: Using benzoic acid as a preservative in controlled amounts.
- Research: Conducting experiments with accurate measurements.
Quick Checklist for Calculating Molar Mass

- Step 1: Identify the chemical formula (C7H6O3).
- Step 2: Look up atomic masses (C, H, O).
- Step 3: Multiply atomic masses by the number of atoms.
- Step 4: Add the results to get the total molar mass.
Final Thoughts

Calculating the molar mass of C7H6O3 is a fundamental skill in chemistry that ensures precision in scientific work. By following the steps outlined above, you can easily determine its molar mass as 138.13 g/mol. This knowledge is invaluable for students, researchers, and professionals alike.
What is the molar mass of C7H6O3?
+The molar mass of C7H6O3 is 138.13 g/mol.
Why is the molar mass of C7H6O3 important?
+It is crucial for stoichiometry, chemical reactions, and precise measurements in industries like pharmaceuticals and food preservation.
How do you calculate the molar mass of a compound?
+Multiply the atomic mass of each element by its number of atoms in the formula and sum the results.
molar mass calculation,chemical formula,atomic mass,stoichiometry,benzoic acid,methylbenzoate,pharmaceuticals,food preservation,laboratory experiments.