Understanding N2O Resonance Hybrid Structure Simplified

Nitrous oxide (N2O), commonly known as laughing gas, is a fascinating molecule with a unique structure that often puzzles students and chemistry enthusiasts alike. Understanding its resonance hybrid structure is crucial for grasping its chemical behavior and applications. In this post, we’ll break down the concept in simple terms, ensuring clarity for both informational and commercial-intent audiences.
What is N2O Resonance Hybrid Structure?

The N2O resonance structure refers to the delocalized electrons within the molecule, which cannot be represented by a single Lewis structure. Instead, N2O exists as a hybrid of multiple resonance forms, contributing to its stability and unique properties. This concept is essential in fields like chemistry education, research, and industrial applications, such as N2O in medical use or automotive performance enhancement.
Key Components of N2O Resonance Structure
To understand N2O, let’s break it down into its key components:
- Nitrogen Atoms (N): Two nitrogen atoms form the core of the molecule.
- Oxygen Atom (O): A single oxygen atom bonds with one of the nitrogen atoms.
- Electron Delocalization: Electrons are shared across the molecule, creating a stable resonance hybrid.
How to Draw N2O Resonance Structures
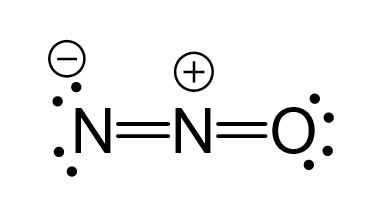
Drawing the resonance structures of N2O involves the following steps:
- Start with the basic Lewis structure, showing all atoms and their valence electrons.
- Identify possible locations for double and single bonds between nitrogen and oxygen.
- Draw all possible resonance forms, ensuring the octet rule is satisfied for each atom.
- Combine these forms into a single resonance hybrid structure.
📌 Note: Resonance structures do not exist individually but contribute to the overall hybrid structure of the molecule.
Applications of N2O Resonance Hybrid Structure

Understanding N2O’s resonance hybrid structure has practical applications in various fields:
| Field | Application |
|---|---|
| Medicine | Used as an anesthetic and analgesic in dental and surgical procedures. |
| Automotive | Acts as a performance enhancer in internal combustion engines. |
| Food Industry | Employed as a propellant in aerosol cans for whipped cream. |

Checklist for Mastering N2O Resonance Hybrid Structure

Here’s a quick checklist to ensure you grasp the concept:
- Understand the basics of Lewis structures and resonance theory.
- Practice drawing all possible resonance forms of N2O.
- Analyze the electron delocalization in the hybrid structure.
- Explore real-world applications of N2O in medicine, automotive, and food industries.
By mastering the N2O resonance hybrid structure, you’ll gain valuable insights into its chemical behavior and applications. Whether you’re a student, researcher, or industry professional, this knowledge is essential for advancing in chemistry-related fields. (N2O structure, resonance theory, chemical bonding)
What is the importance of N2O resonance hybrid structure?
+
The resonance hybrid structure explains N2O’s stability and reactivity, making it crucial for understanding its applications in medicine, automotive, and other industries.
How many resonance structures does N2O have?
+
N2O has three main resonance structures, which combine to form its hybrid structure.
Can N2O be used in everyday products?
+
Yes, N2O is used in products like whipped cream cans and as a medical anesthetic, showcasing its versatility.



