NH3 Bonding Angle: Unlocking Ammonia's Molecular Secrets

Ammonia (NH3) is a fundamental molecule with a unique structure that has intrigued chemists for decades. At the heart of its molecular geometry lies the NH3 bonding angle, a critical factor in understanding its properties and applications. This angle, approximately 107.3 degrees, is a result of the interplay between bonding and lone pair electrons, showcasing the fascinating world of molecular interactions. Whether you’re a student, researcher, or industry professional, grasping the nuances of the NH3 bonding angle is essential for unlocking ammonia’s potential in various fields, from agriculture to energy storage.
Understanding the NH3 Bonding Angle
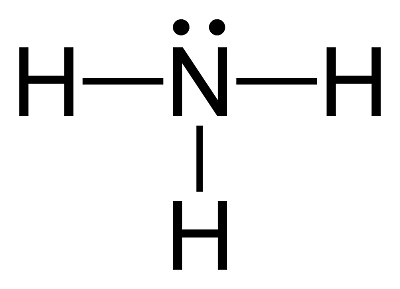
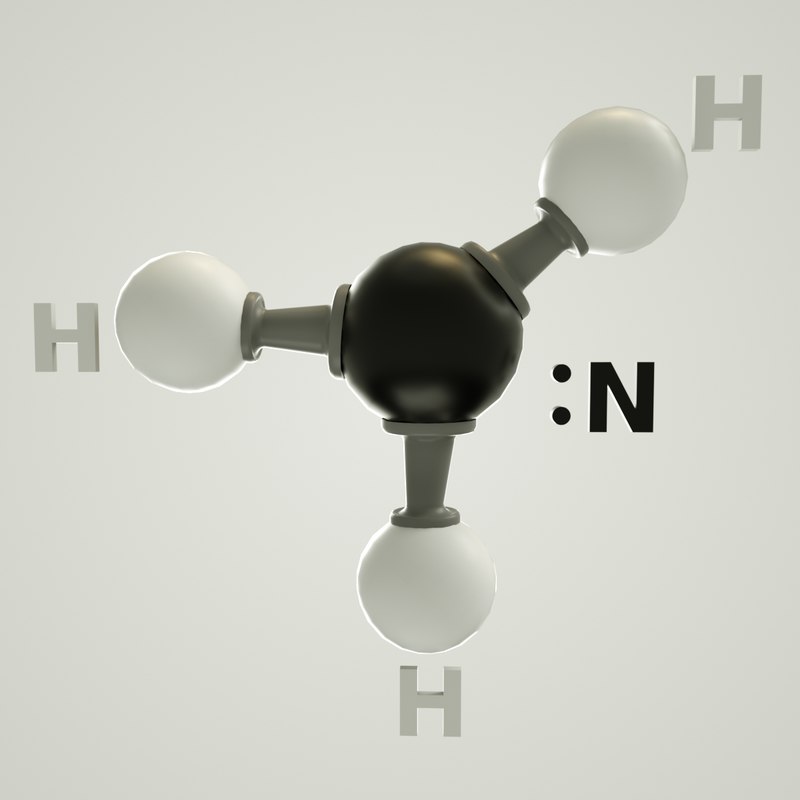
The NH3 bonding angle is a key aspect of ammonia’s molecular structure. Ammonia consists of one nitrogen atom bonded to three hydrogen atoms, with a lone pair of electrons on the nitrogen. According to Valence Shell Electron Pair Repulsion (VSEPR) theory, electron pairs around the central atom repel each other, determining the molecule’s shape. In NH3, the lone pair occupies more space than bonding pairs, causing the H-N-H bond angles to deviate from the ideal tetrahedral angle of 109.5 degrees, resulting in the observed 107.3-degree angle.
💡 Note: The lone pair in NH3 plays a crucial role in reducing the bonding angle, a principle applicable to other molecules with similar electron configurations.
Why the NH3 Bonding Angle Matters
The NH3 bonding angle is not just a theoretical concept; it has practical implications in chemistry and industry. Here’s why it matters:
- Chemical Reactivity: The angle influences how ammonia interacts with other molecules, affecting its reactivity in chemical processes.
- Physical Properties: It determines ammonia’s boiling and melting points, solubility, and other physical characteristics.
- Industrial Applications: Understanding this angle is vital for optimizing ammonia’s use in fertilizers, refrigerants, and emerging technologies like hydrogen storage.
Applications of Ammonia’s Molecular Structure
Ammonia’s unique bonding angle makes it a versatile compound in various industries:
| Application | Relevance of NH3 Bonding Angle |
|---|---|
| Fertilizers | The angle affects ammonia’s ability to form ammonium compounds, essential for plant nutrition. |
| Refrigeration | Ammonia’s physical properties, influenced by its structure, make it an efficient refrigerant. |
| Hydrogen Storage | The bonding angle impacts ammonia’s potential as a hydrogen carrier in clean energy solutions. |
🔍 Note: For commercial applications, optimizing the NH3 bonding angle can enhance efficiency and reduce costs.
How to Analyze NH3 Bonding Angle
For those interested in studying or applying ammonia’s molecular structure, here’s a checklist:
- Use VSEPR Theory: Apply the theory to predict the bonding angle based on electron pair arrangements.
- Leverage Spectroscopy: Techniques like infrared (IR) and Raman spectroscopy can provide insights into molecular geometry.
- Consult Computational Tools: Software like Gaussian or Spartan can simulate and analyze NH3’s structure.
Final Thoughts

The NH3 bonding angle is more than just a number; it’s a gateway to understanding ammonia’s molecular secrets. From its role in chemical reactivity to its applications in industry, this angle highlights the importance of molecular geometry in science and technology. Whether you’re exploring ammonia for academic research or commercial purposes, mastering this concept will undoubtedly enhance your understanding and innovation.
What causes the NH3 bonding angle to be 107.3 degrees?
+The lone pair of electrons on the nitrogen atom repels the bonding pairs more strongly, reducing the H-N-H angle from the ideal tetrahedral 109.5 degrees to 107.3 degrees.
How does the NH3 bonding angle affect its solubility?
+The bonding angle influences ammonia’s polarity and hydrogen bonding capabilities, making it highly soluble in water and other polar solvents.
Can the NH3 bonding angle be altered?
+While the angle is inherently determined by molecular structure, external conditions like pressure or complex formation can subtly influence it.
ammonia structure,molecular geometry,VSEPR theory,chemical bonding,industrial applications,hydrogen storage,fertilizers,refrigeration,spectroscopy,computational chemistry.



