Understanding NH3 Oxidation State: A Comprehensive Guide

Understanding the NH3 oxidation state is crucial for anyone working in chemistry, environmental science, or industrial applications. Ammonia (NH3) plays a significant role in various processes, from fertilizer production to fuel cells. This guide will break down the concept of NH3 oxidation state, its importance, and how it impacts different fields. Whether you're a student, researcher, or professional, this comprehensive overview will provide the clarity you need. (NH3 oxidation state, ammonia chemistry, oxidation state basics)
What is the NH3 Oxidation State?

The oxidation state of an atom in a molecule indicates its degree of oxidation. For NH3 (ammonia), understanding the oxidation state of nitrogen (N) is key. In NH3, nitrogen is in the -3 oxidation state. This is because nitrogen gains three electrons when forming bonds with hydrogen atoms. (oxidation state definition, nitrogen in NH3, ammonia molecule structure)
Why is NH3 Oxidation State Important?
The NH3 oxidation state is vital in chemical reactions, particularly in processes like ammonia oxidation and its role in the nitrogen cycle. It also influences applications in catalysis, fuel cells, and pollution control. Understanding this concept helps predict reactivity and optimize industrial processes. (ammonia oxidation, nitrogen cycle, industrial applications)
How to Determine NH3 Oxidation State
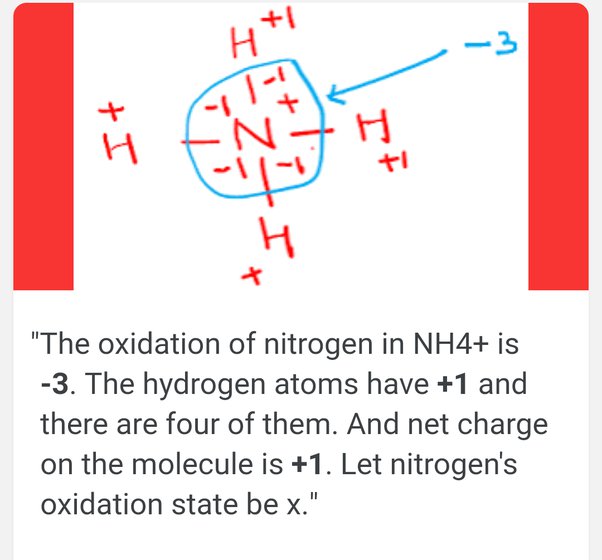
To determine the oxidation state of nitrogen in NH3, follow these steps:
- Hydrogen typically has an oxidation state of +1.
- The sum of oxidation states in a neutral molecule is zero.
- Let N be the oxidation state of nitrogen. The equation is: N + 3(+1) = 0, solving for N gives -3.
💡 Note: This method applies to neutral molecules. Charged species require adjustments based on the overall charge. (oxidation state calculation, chemical equations, ammonia properties)
Applications of NH3 Oxidation State

The NH3 oxidation state is relevant in several areas:
| Application | Relevance |
|---|---|
| Fertilizer Production | Ammonia oxidation is key in producing nitrogen-based fertilizers. |
| Environmental Science | Understanding NH3 oxidation helps study air pollution and the nitrogen cycle. |
| Fuel Cells | NH3 is a potential fuel source, and its oxidation state impacts efficiency. |
(fertilizer production, environmental science, fuel cell technology)
In summary, the NH3 oxidation state is a fundamental concept with wide-ranging applications. By understanding nitrogen’s -3 oxidation state in ammonia, you can better grasp its role in chemical reactions and industrial processes. Whether you're studying chemistry or working in a related field, this knowledge is invaluable. (NH3 oxidation state, ammonia applications, chemical fundamentals)
What is the oxidation state of nitrogen in NH3?
+The oxidation state of nitrogen in NH3 is -3.
Why is NH3 oxidation important in environmental science?
+NH3 oxidation plays a critical role in the nitrogen cycle and air pollution studies, helping scientists understand environmental impacts.
How does NH3 oxidation state affect fuel cells?
+The oxidation state of NH3 influences its reactivity and efficiency as a fuel source in fuel cell technology.


