Understanding O2 Electron Configuration: A Quick Guide

Understanding the O2 electron configuration is essential for anyone studying chemistry or physics. Oxygen (O2) is a fundamental element with a unique electron arrangement that plays a crucial role in various natural and industrial processes. This quick guide will break down the electron configuration of O2, explain its significance, and provide practical insights for both informational and commercial audiences. Whether you're a student, researcher, or industry professional, this post will help you grasp the basics and apply them effectively.
What is O2 Electron Configuration?

The electron configuration of oxygen (O2) refers to how electrons are distributed in its atomic orbitals. Oxygen has an atomic number of 8, meaning it has 8 electrons. In its ground state, the electron configuration of a single oxygen atom is 1s² 2s² 2p⁴. When two oxygen atoms bond to form O2, the electron configuration adjusts to accommodate the molecular structure. This results in a unique arrangement that explains O2’s reactivity and properties.
How to Determine O2 Electron Configuration
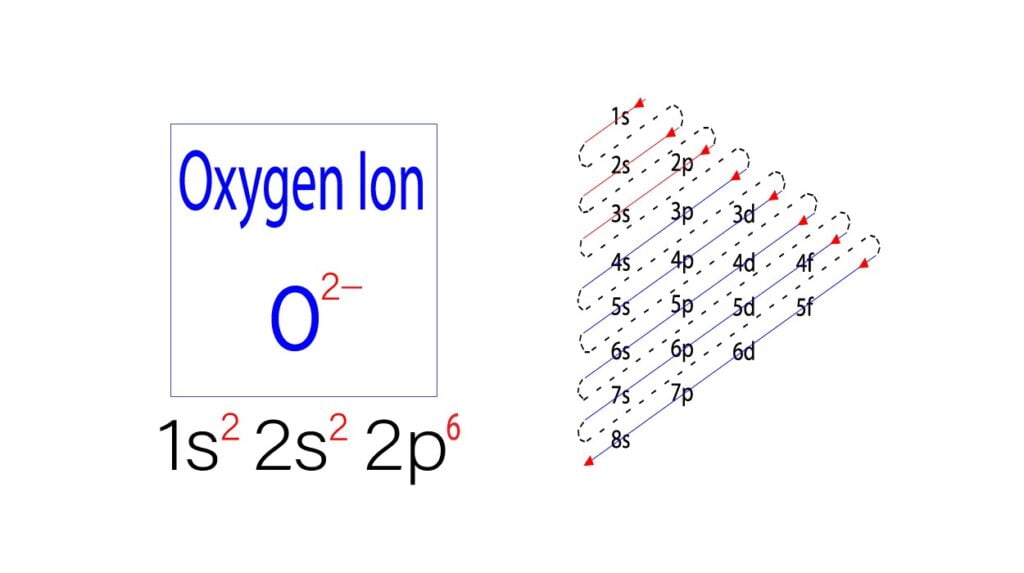
To understand the O2 electron configuration, follow these steps:
- Step 1: Identify the Atomic Configuration – Start with the electron configuration of a single oxygen atom: 1s² 2s² 2p⁴.
- Step 2: Consider Molecular Bonding – In O2, two oxygen atoms share electrons to form a double bond, resulting in a molecular orbital configuration.
- Step 3: Apply Molecular Orbital Theory – Use molecular orbital theory to determine the final electron arrangement in O2, which is (σ1s)² (σ*1s)² (σ2s)² (σ*2s)² (σ2p)² (π2p)⁴ (π*2p)².
📌 Note: Molecular orbital theory is crucial for accurately describing the electron configuration of diatomic molecules like O2.
Importance of O2 Electron Configuration

The electron configuration of O2 is vital for several reasons:
- Reactivity – It explains why oxygen is highly reactive and forms bonds with other elements.
- Biological Processes – O2’s electron configuration is central to cellular respiration in living organisms.
- Industrial Applications – Understanding O2’s configuration aids in optimizing processes like combustion and oxidation in industries.
Practical Applications of O2 Electron Configuration

For commercial audiences, knowing the O2 electron configuration can enhance:
- Chemical Manufacturing – Improve efficiency in producing oxygen-based compounds.
- Environmental Science – Analyze oxygen’s role in air quality and pollution control.
- Medical Technology – Develop oxygen therapy devices and respiratory equipment.
Quick Checklist for O2 Electron Configuration

- Understand the atomic electron configuration of oxygen: 1s² 2s² 2p⁴.
- Apply molecular orbital theory to determine O2’s configuration.
- Recognize the role of O2’s electron arrangement in reactivity and bonding.
- Explore practical applications in industries like healthcare and manufacturing.
Mastering the O2 electron configuration opens doors to understanding its role in science and industry. By following this guide, you’ll gain a solid foundation to apply this knowledge effectively. Whether for academic research or commercial innovation, the electron configuration of O2 is a key concept worth exploring further. (electron configuration, molecular orbital theory, oxygen reactivity)
What is the electron configuration of a single oxygen atom?
+
The electron configuration of a single oxygen atom is 1s² 2s² 2p⁴.
How does O2’s electron configuration differ from a single oxygen atom?
+
O2’s electron configuration involves molecular orbitals due to the bonding of two oxygen atoms, unlike the atomic configuration of a single oxygen atom.
Why is O2’s electron configuration important in biology?
+
O2’s electron configuration is crucial in biology as it enables oxygen to participate in cellular respiration, providing energy for living organisms.