Master Regents Chem: Essential Reference Table Guide
<!DOCTYPE html>
Are you preparing for the Regents Chemistry exam and feeling overwhelmed by the vast amount of information? The Regents Chemistry Reference Table is your ultimate ally in mastering key concepts and acing the exam. This guide will walk you through the essentials of the reference table, ensuring you know exactly what to focus on. Whether you’re a student or a teacher, this resource is designed to simplify your study process and boost your confidence. (Regents Chemistry, Reference Table Guide, Study Tips)
Why the Regents Chemistry Reference Table is Crucial

The Regents Chemistry Reference Table is a comprehensive tool provided during the exam, containing vital data such as element properties, gas laws, and chemical equations. Understanding how to use it effectively can save you time and prevent errors. Here’s why it’s indispensable:
- Quick Access to Data: No need to memorize every detail; the table has you covered.
- Problem-Solving Efficiency: Simplify complex problems by referencing accurate values.
- Exam Confidence: Knowing how to use the table reduces anxiety and improves performance.
📌 Note: Familiarize yourself with the table’s layout before the exam to use it efficiently.
Key Sections of the Regents Chemistry Reference Table

The reference table is divided into several sections, each serving a specific purpose. Here’s a breakdown of the most important ones:
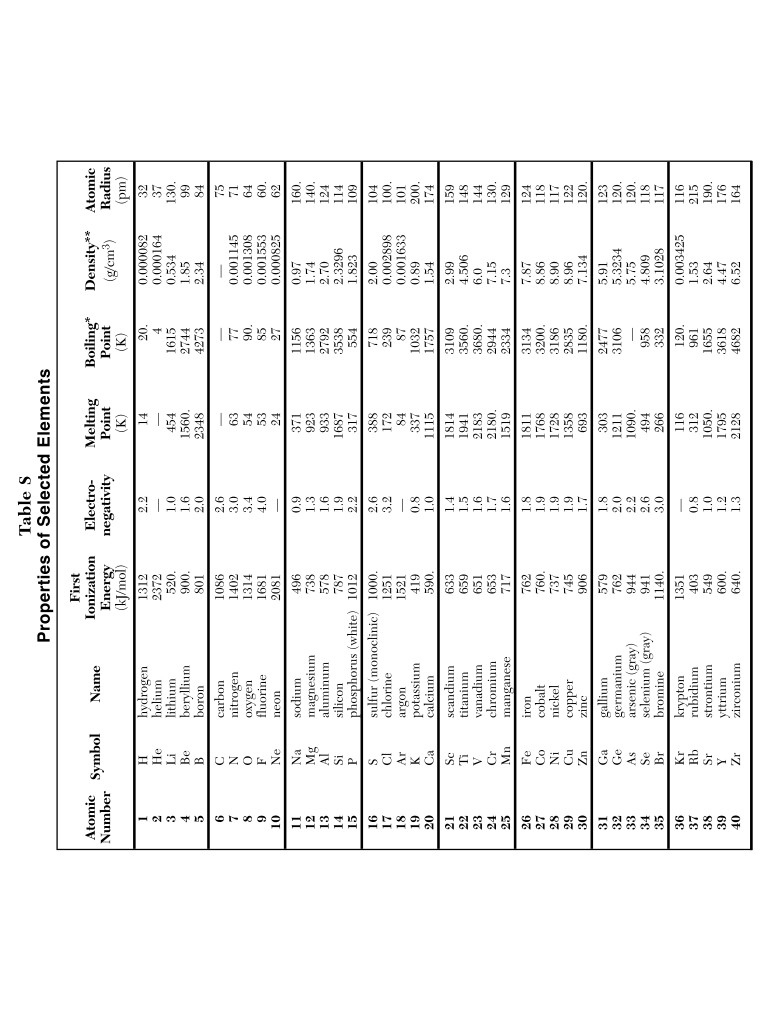
1. Periodic Table of Elements
This section includes atomic numbers, symbols, and masses. It’s essential for identifying elements and understanding their properties. (Periodic Table, Atomic Mass, Element Identification)
2. Common Polyatomic Ions
A quick reference for ions like sulfate (SO₄²⁻) and ammonium (NH₄⁺), crucial for balancing equations and identifying compounds. (Polyatomic Ions, Chemical Compounds)
3. Solubility Guidelines
Determine the solubility of various compounds in water, a frequent topic in exam questions. (Solubility Rules, Chemical Reactions)
| Section | Purpose |
|---|---|
| Periodic Table | Element Identification |
| Polyatomic Ions | Compound Identification |
| Solubility Guidelines | Reaction Predictions |

How to Use the Reference Table Effectively

Maximizing the utility of the reference table requires practice and strategy. Follow these steps:
- Study the Layout: Understand where each section is located for quick access.
- Practice Problems: Use the table during practice exams to simulate real conditions.
- Cross-Reference Data: Combine information from multiple sections for complex problems.
📌 Note: Avoid over-relying on the table; understand the underlying concepts for better retention.
Essential Tips for Regents Chemistry Success

Beyond the reference table, here are additional tips to excel in your Regents Chemistry exam:
- Consistent Practice: Regularly solve past exam questions to reinforce learning.
- Concept Mastery: Focus on understanding topics like stoichiometry and thermodynamics.
- Time Management: Allocate time wisely during the exam to tackle all sections.
In summary, the Regents Chemistry Reference Table is a powerful tool that, when used correctly, can significantly enhance your exam performance. By understanding its sections, practicing regularly, and mastering key concepts, you’ll be well-prepared to tackle any question. Remember, success in Regents Chemistry is about both knowledge and strategy. (Regents Chemistry Prep, Exam Strategies, Chemistry Success)
What is the Regents Chemistry Reference Table?
+The Regents Chemistry Reference Table is a comprehensive resource provided during the exam, containing essential data like the periodic table, solubility rules, and polyatomic ions.
How can I practice using the reference table?
+Use past Regents Chemistry exams and practice problems, incorporating the reference table to simulate real exam conditions.
Are there any sections of the table I should focus on more?
+While all sections are important, focus on the periodic table, solubility guidelines, and polyatomic ions, as they are frequently used in exam questions.



