Mastering Lewis Dot Structures: Essential Rules Simplified

Understanding Lewis Dot Structures is crucial for anyone studying chemistry, as they provide a clear visual representation of the distribution of electrons in molecules. By mastering these structures, you can predict molecular geometry, bond formation, and chemical properties with ease. Whether you're a student preparing for exams or a professional in the field, this guide simplifies the essential rules, making it easier to draw accurate Lewis Dot Structures. Let’s dive into the key principles and step-by-step instructions to enhance your chemical knowledge, (Lewis Dot Structures, Chemical Bonding, Electron Configuration).
What Are Lewis Dot Structures?
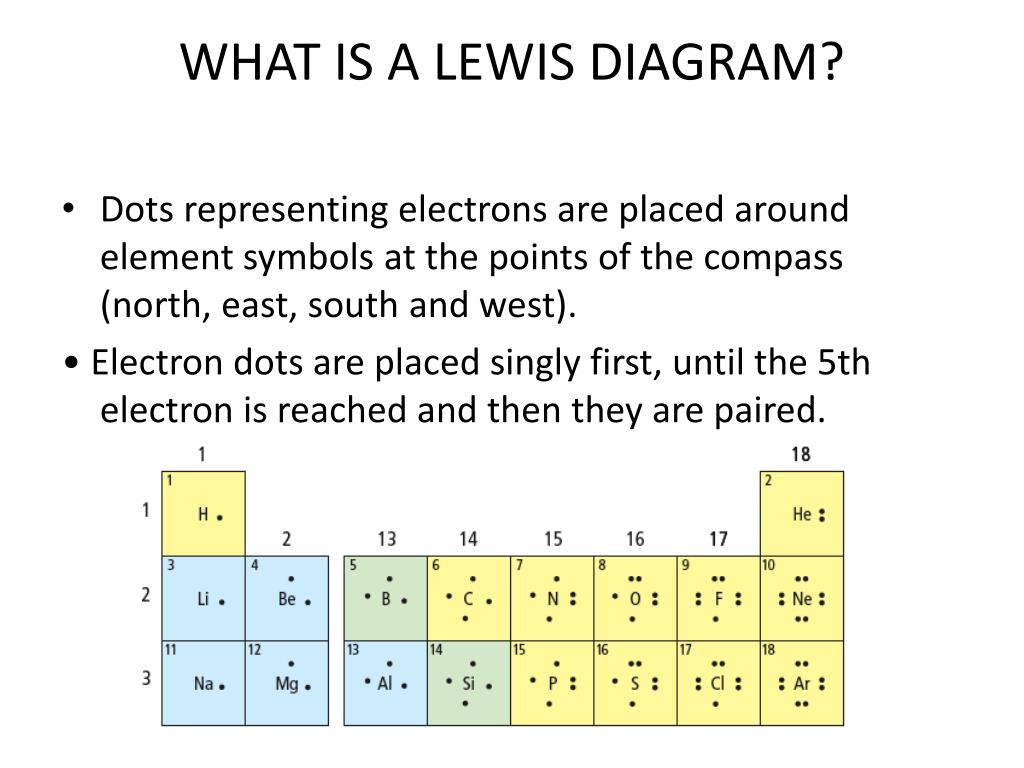
Lewis Dot Structures, also known as electron dot diagrams, illustrate the valence electrons of atoms within a molecule. They help in understanding how atoms bond and share electrons. Each dot represents a valence electron, and lines between atoms signify covalent bonds. Mastering these structures is fundamental for grasping molecular behavior, (Molecular Geometry, Covalent Bonds, Valence Electrons).
Essential Rules for Drawing Lewis Dot Structures

1. Determine the Total Number of Valence Electrons
Start by identifying the valence electrons of each atom in the molecule. Add them together to find the total number of electrons to distribute, (Valence Electrons, Molecular Structure, Electron Distribution).
2. Identify the Central Atom
The central atom is usually the least electronegative element in the molecule. It serves as the core around which other atoms bond, (Central Atom, Electronegativity, Molecular Core).
3. Connect Atoms with Single Bonds
Place single bonds between the central atom and surrounding atoms. Each bond represents two electrons, (Single Bonds, Covalent Bonding, Molecular Connectivity).
Example:
| Molecule | Central Atom | Surrounding Atoms |
|---|---|---|
| Water (H₂O) | Oxygen (O) | Hydrogen (H) |

📌 Note: Always prioritize the central atom's stability when forming bonds.
4. Distribute Remaining Electrons
Place remaining electrons as lone pairs on the outer atoms first, ensuring each atom satisfies the octet rule (except hydrogen, which needs two electrons), (Octet Rule, Lone Pairs, Electron Stability).
5. Check for Satisfying the Octet Rule
Ensure all atoms (except hydrogen) have eight valence electrons. If not, form double or triple bonds as needed, (Double Bonds, Triple Bonds, Octet Rule Exceptions).
Common Exceptions and Advanced Tips

Not all molecules follow the octet rule. Some, like boron or beryllium compounds, have fewer than eight electrons. Others, such as phosphorus or sulfur, can expand their octets. Understanding these exceptions is key to mastering Lewis Dot Structures, (Octet Rule Exceptions, Expanded Octets, Incomplete Octets).
Checklist: Steps to Draw Lewis Dot Structures

- Count total valence electrons.
- Identify the central atom.
- Connect atoms with single bonds.
- Distribute remaining electrons as lone pairs.
- Check and adjust for the octet rule.
Mastering Lewis Dot Structures is a foundational skill in chemistry that simplifies understanding molecular interactions. By following these essential rules and tips, you’ll be able to draw accurate structures with confidence. Practice regularly to reinforce your knowledge and tackle complex molecules effortlessly, (Chemical Structures, Molecular Interactions, Chemistry Basics).
What is the octet rule in Lewis Dot Structures?
+
The octet rule states that atoms tend to gain, lose, or share electrons to achieve eight valence electrons, ensuring stability, (Octet Rule, Electron Stability, Chemical Bonding).
How do I handle molecules with expanded octets?
+
Elements in period 3 or higher, like phosphorus or sulfur, can have more than eight electrons. Distribute extra electrons as lone pairs or multiple bonds, (Expanded Octets, Electron Distribution, Molecular Exceptions).
Why is the central atom important in Lewis structures?
+
The central atom serves as the core for bonding and determines the molecule’s overall shape and electron distribution, (Central Atom, Molecular Shape, Electron Configuration).



