SCN Lewis Structure: Best Representation Guide

Understanding the SCN Lewis Structure is crucial for anyone studying chemistry, especially when dealing with coordination compounds and chemical bonding. Thiocyanate (SCN⁻) is a polyatomic ion that plays a significant role in various chemical reactions and biological processes. This guide will walk you through the best representation of the SCN Lewis structure, ensuring clarity and accuracy in your chemical drawings.
What is the SCN Lewis Structure?
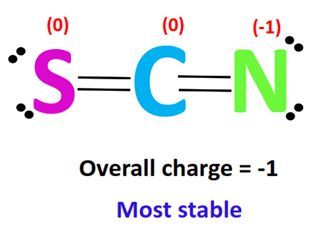
The SCN Lewis structure represents the arrangement of atoms and electrons in the thiocyanate ion (SCN⁻). It consists of sulfur (S), carbon ©, and nitrogen (N) atoms, with a negative charge distributed across the ion. Mastering this structure is essential for predicting molecular geometry, polarity, and reactivity.
Step-by-Step Guide to Drawing the SCN Lewis Structure
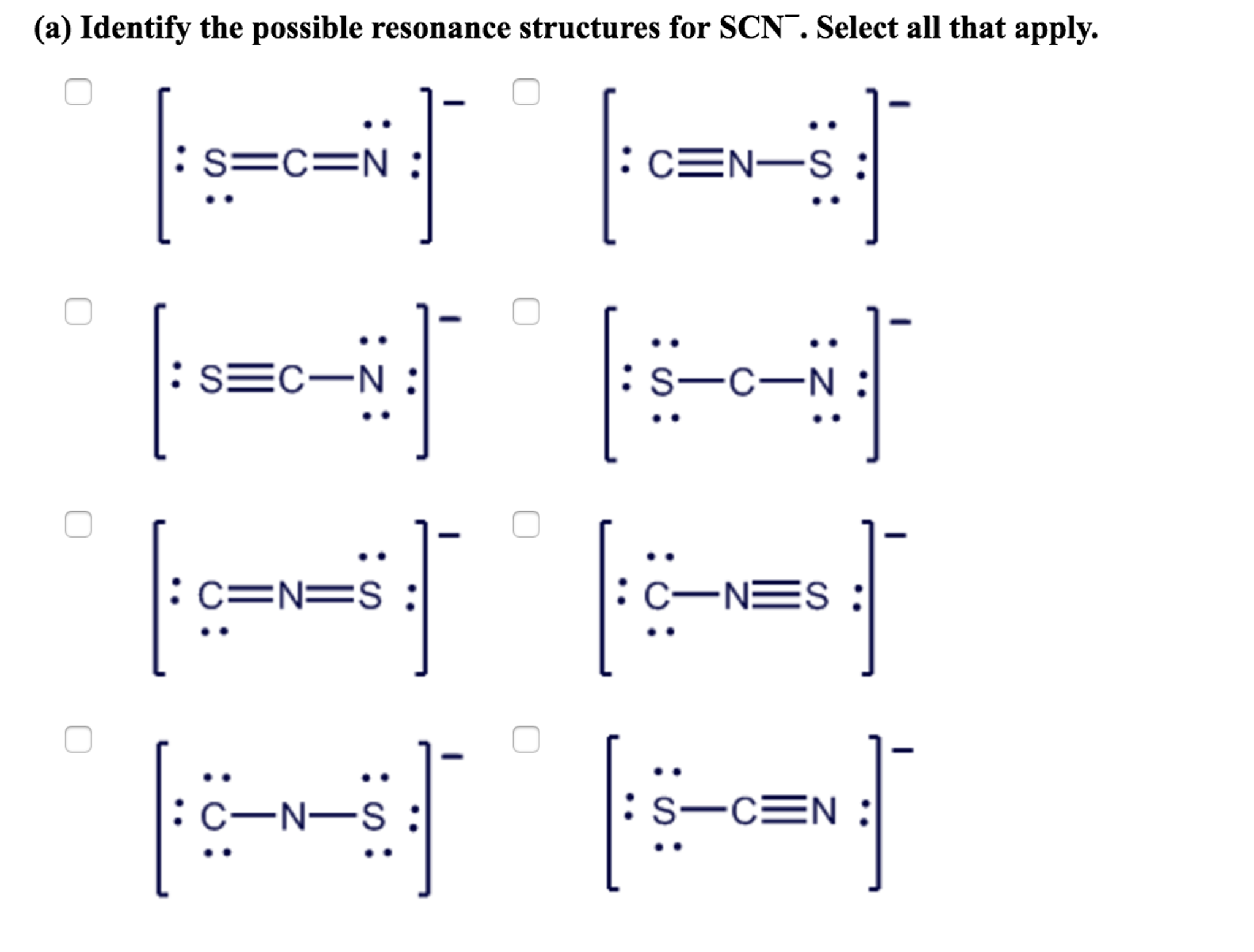
1. Determine the Total Number of Valence Electrons
- Sulfur (S): 6 valence electrons
- Carbon ©: 4 valence electrons
- Nitrogen (N): 5 valence electrons
- Negative Charge: 1 additional electron
Total: 6 + 4 + 5 + 1 = 16 valence electrons
2. Identify the Central Atom
Carbon © is the central atom due to its lower electronegativity compared to S and N.
3. Draw the Skeletal Structure
Connect the atoms with single bonds: S-C-N.
4. Distribute Remaining Electrons
- Place lone pairs on S and N to complete their octets.
- Remaining electrons are distributed as follows:
- S: 2 lone pairs
- N: 1 lone pair
- S: 2 lone pairs
5. Check Formal Charges
Ensure the formal charges are minimized:
- S: 6 – (2 + 2) = +2
- C: 4 – (4) = 0
- N: 5 – (2 + 3) = -1
To balance the charges, form a double bond between C and N, resulting in:
- S: 6 – (2 + 2) = +1
- C: 4 – (2 + 2) = 0
- N: 5 – (4 + 1) = 0
📌 Note: The double bond between C and N is the most stable configuration for SCN⁻.
Key Takeaways for SCN Lewis Structure
- Central Atom: Carbon ©
- Bonding: S-C single bond, C=N double bond
- Lone Pairs: 2 on S, 1 on N
- Formal Charges: +1 on S, 0 on C and N
Why is the SCN Lewis Structure Important?
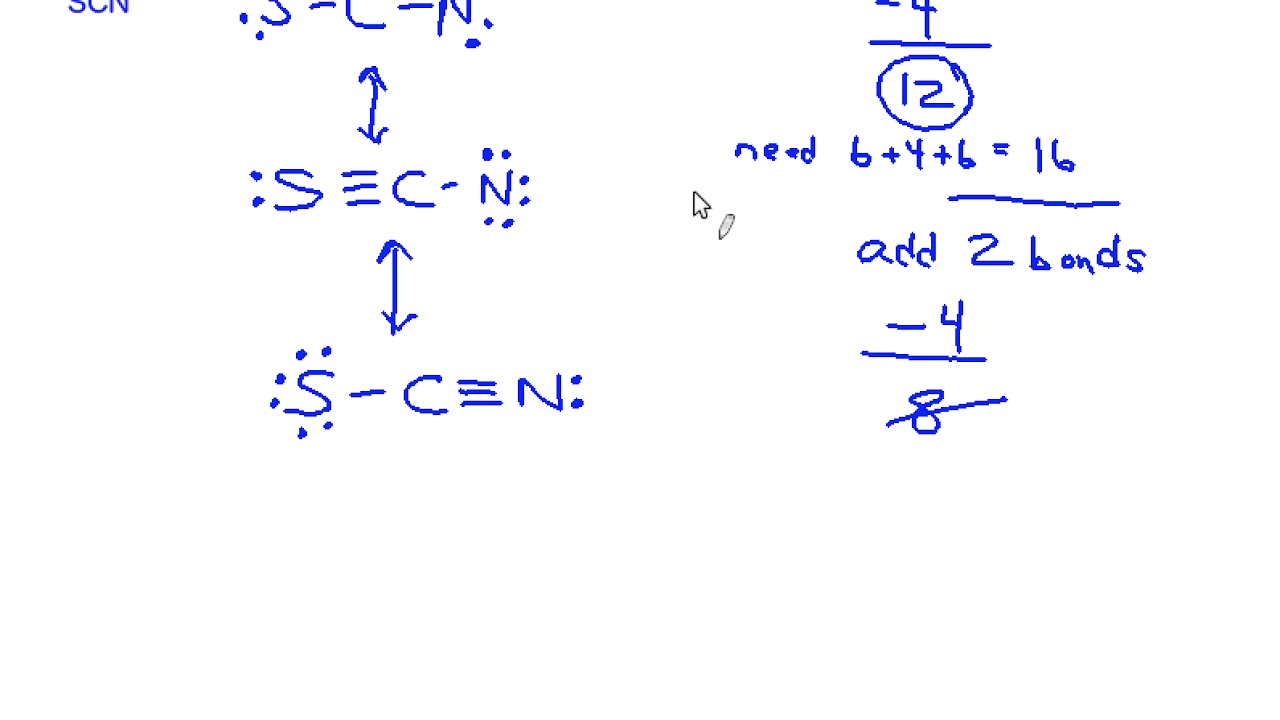
The SCN Lewis structure is vital for understanding its role in chemical reactions, such as coordination with metal ions and its use in analytical chemistry. It also helps predict properties like polarity and solubility.
Checklist for Drawing the SCN Lewis Structure
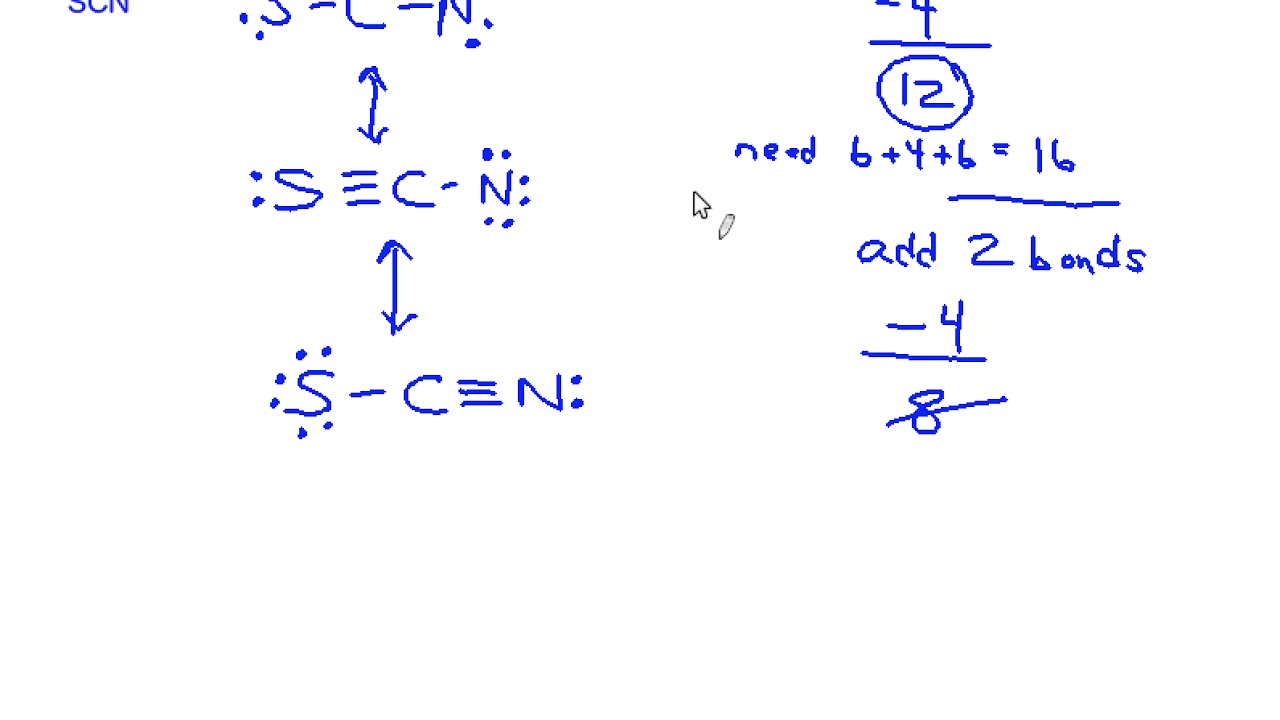
- Count total valence electrons (16).
- Place Carbon as the central atom.
- Draw single bonds between S-C-N.
- Distribute remaining electrons as lone pairs.
- Adjust bonds to minimize formal charges.
(SCN Lewis Structure,Thiocyanate Ion,Chemical Bonding,Molecular Geometry,Coordination Compounds)
What is the SCN Lewis structure?
+The SCN Lewis structure consists of sulfur (S) bonded to carbon (C) via a single bond, and carbon double-bonded to nitrogen (N), with lone pairs on S and N.
Why is the double bond between C and N important?
+The double bond minimizes formal charges, making the structure more stable and accurate for representing the thiocyanate ion.
How does the SCN ion participate in coordination compounds?
+The SCN⁻ ion acts as a ligand, donating its lone pair of electrons to form coordinate covalent bonds with metal ions.
Understanding the SCN Lewis structure is fundamental for both academic and practical applications in chemistry. By following this guide, you’ll be able to accurately represent the thiocyanate ion and apply this knowledge to more complex chemical concepts. Whether you’re a student or a professional, mastering this structure will enhance your chemical understanding and problem-solving skills.
(SCN Lewis Structure,Thiocyanate Ion,Chemical Bonding,Molecular Geometry,Coordination Compounds)


