Understanding SCN Formal Charge: A Quick Guide

Understanding SCN Formal Charge: A Quick Guide
In the world of chemistry, grasping the concept of formal charge is crucial, especially when dealing with complex molecules like thiocyanate (SCN). Whether you're a student, researcher, or simply curious about chemical principles, this guide will walk you through the essentials of SCN formal charge calculation and its significance. (SCN formal charge, thiocyanate formal charge, chemical bonding)
What is SCN Formal Charge?

The formal charge of an atom in a molecule is a theoretical charge assigned to it based on the assumption that electrons in all chemical bonds are shared equally. For the thiocyanate ion (SCN−), understanding the formal charge helps in predicting its reactivity and stability. (formal charge definition, thiocyanate ion, chemical stability)
How to Calculate SCN Formal Charge
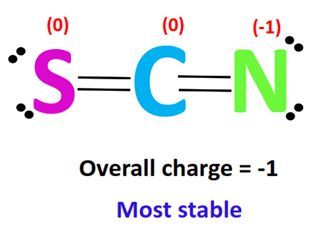
Calculating the formal charge involves a straightforward formula:
Formal Charge = Valence Electrons - (Non-bonding Electrons + Number of Bonds)
Step-by-Step Calculation for SCN
- Sulfur (S): 6 valence electrons, 2 non-bonding electrons, 2 bonds → Formal Charge = 6 - (2 + 2) = 2
- Carbon (C): 4 valence electrons, 0 non-bonding electrons, 3 bonds → Formal Charge = 4 - (0 + 3) = 1
- Nitrogen (N): 5 valence electrons, 2 non-bonding electrons, 2 bonds → Formal Charge = 5 - (2 + 2) = 1
The overall charge of SCN− is -1, which is distributed among the atoms. (formal charge calculation, thiocyanate structure, electron distribution)
Importance of SCN Formal Charge

Understanding the formal charge of SCN is vital for several reasons:
- Predicting Reactivity: It helps in determining how SCN− might react in different chemical environments.
- Stability Analysis: A balanced formal charge distribution indicates a more stable molecule.
- Educational Value: It’s a fundamental concept in chemistry education, aiding in the understanding of molecular structures. (chemical reactivity, molecular stability, chemistry education)
Practical Applications of SCN Formal Charge

The knowledge of SCN formal charge is applied in various fields:
- Analytical Chemistry: Used in identifying and quantifying thiocyanate ions in solutions.
- Environmental Science: Helps in studying the impact of thiocyanate ions on ecosystems.
- Pharmaceutical Research: Important in drug development involving thiocyanate-based compounds. (analytical chemistry, environmental science, pharmaceutical research)
📌 Note: Always double-check your calculations to ensure accuracy in determining formal charges.
Mastering the concept of SCN formal charge not only enhances your understanding of chemical principles but also opens doors to practical applications in various scientific disciplines. By following the steps outlined in this guide, you can confidently calculate and interpret formal charges, contributing to your expertise in chemistry. (chemical principles, scientific disciplines, chemistry expertise)
What is the formal charge of the sulfur atom in SCN−?
+The formal charge of the sulfur atom in SCN− is typically +1, considering the standard resonance structure.
Why is formal charge important in chemistry?
+Formal charge helps in understanding the distribution of electrons in a molecule, which is crucial for predicting reactivity and stability.
Can the formal charge of an atom be zero?
+Yes, if the number of valence electrons equals the sum of non-bonding electrons and bonds, the formal charge will be zero.


