Understanding sp3 Hybridized Carbon: A Simple Guide

<!DOCTYPE html>
Carbon is one of the most versatile elements in chemistry, and its ability to form various types of bonds is crucial in organic compounds. Among the different hybridization states, sp3 hybridized carbon is particularly important due to its role in forming tetrahedral structures. This guide will break down the concept of sp3 hybridization, its significance, and its applications in a simple, easy-to-understand manner. (carbon hybridization, organic chemistry, molecular structure)
What is sp3 Hybridized Carbon?

In chemistry, hybridization refers to the mixing of atomic orbitals to form new hybrid orbitals with specific geometries. sp3 hybridization occurs when one s orbital and three p orbitals of a carbon atom combine to form four equivalent sp3 hybrid orbitals. These orbitals are arranged tetrahedrally around the carbon atom, with bond angles of approximately 109.5 degrees. (atomic orbitals, tetrahedral geometry, bond angles)
How Does sp3 Hybridization Occur?

To understand sp3 hybridization, let’s break down the process:
- Step 1: Orbital Mixing – The s orbital and three p orbitals of carbon mix to form four sp3 hybrid orbitals.
- Step 2: Tetrahedral Arrangement – These hybrid orbitals arrange themselves in a tetrahedral geometry, maximizing stability.
- Step 3: Bond Formation – Each sp3 hybrid orbital can overlap with orbitals from other atoms to form strong, single covalent bonds. (covalent bonds, orbital overlap, molecular stability)
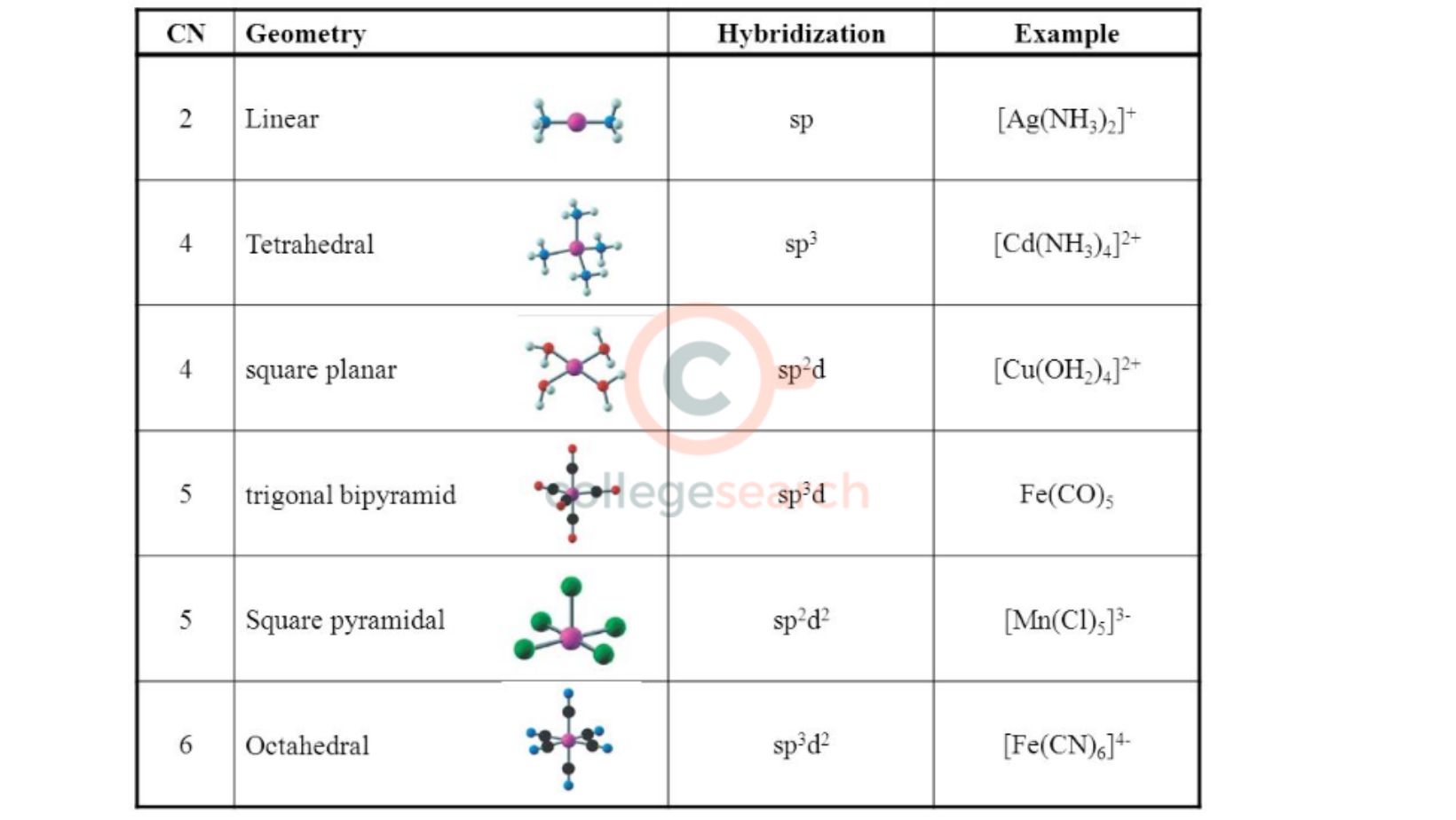
Key Characteristics of sp3 Hybridized Carbon

| Characteristic | Description |
|---|---|
| Geometry | Tetrahedral |
| Bond Angle | 109.5 degrees |
| Number of Bonds | Four single bonds |
| Example | Methane (CH₄) |
Applications of sp3 Hybridized Carbon

sp3 hybridized carbon is fundamental in various fields, including:
- Organic Chemistry – Forms the basis of alkanes and many organic compounds.
- Biochemistry – Essential in the structure of biomolecules like proteins and carbohydrates.
- Material Science – Used in the synthesis of polymers and other materials. (organic compounds, biomolecules, polymers)
💡 Note: sp3 hybridization is not limited to carbon; other elements like silicon and germanium can also exhibit similar hybridization states.
Checklist for Understanding sp3 Hybridization
- Know the definition of sp3 hybridization.
- Understand the tetrahedral geometry and bond angles.
- Identify examples of sp3 hybridized compounds.
- Recognize its applications in chemistry and related fields. (hybridization definition, tetrahedral geometry, chemical applications)
In summary, sp3 hybridized carbon is a cornerstone of organic chemistry, enabling the formation of stable, tetrahedral structures. By understanding its principles and applications, you can gain deeper insights into the molecular world and its impact on various scientific disciplines. Whether you're a student, researcher, or enthusiast, mastering sp3 hybridization is a valuable step in your chemical journey. (organic chemistry, molecular structure, chemical principles)
What is the bond angle in sp3 hybridization?
+The bond angle in sp3 hybridization is approximately 109.5 degrees, forming a tetrahedral geometry.
What is an example of sp3 hybridized carbon?
+Methane (CH₄) is a classic example of a molecule with sp3 hybridized carbon.
How does sp3 hybridization differ from sp2 and sp hybridization?
+sp3 hybridization involves four equivalent orbitals forming single bonds, while sp2 and sp involve three and two orbitals, respectively, allowing for double and triple bonds.


