Zinc Ion Electron Configuration: Simplified Guide

Understanding zinc ion electron configuration is crucial for anyone studying chemistry or materials science. Zinc, a transition metal, plays a significant role in various biological and industrial processes. This guide simplifies the electron configuration of zinc ions, making it accessible for both informational and commercial audiences. Whether you’re a student, researcher, or professional, this post will provide valuable insights into zinc ion electron configuration, zinc properties, and its applications.
What is Zinc Ion Electron Configuration?

Zinc (Zn) is a chemical element with the atomic number 30. In its neutral state, zinc’s electron configuration is 1s² 2s² 2p⁶ 3s² 3p⁶ 4s² 3d¹⁰. When zinc loses two electrons to form Zn²⁺, its electron configuration changes. The zinc ion electron configuration becomes 1s² 2s² 2p⁶ 3s² 3p⁶ 3d¹⁰, as the two 4s electrons are removed. This simplified configuration is essential for understanding zinc’s chemical behavior and reactivity.
💡 Note: The removal of the 4s electrons in zinc ions is a key factor in its stability and bonding properties.
Why is Zinc Ion Electron Configuration Important?

The zinc ion electron configuration is vital for several reasons. It explains zinc’s role in biological systems, such as enzyme function and DNA synthesis. In industrial applications, understanding zinc’s electron configuration helps in developing alloys, batteries, and corrosion-resistant materials. For commercial audiences, this knowledge is crucial for product development and material selection.
Key Applications of Zinc Ions
- Biological Systems: Zinc ions act as cofactors for enzymes, supporting essential life processes.
- Industrial Uses: Zinc is used in galvanizing steel, manufacturing batteries, and producing brass.
- Medical Applications: Zinc supplements are used to boost immunity and treat deficiencies.
How to Determine Zinc Ion Electron Configuration
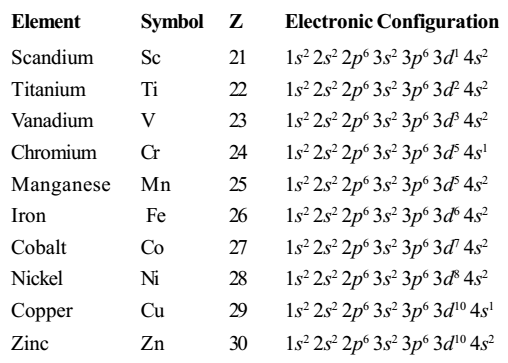
To determine the zinc ion electron configuration, follow these steps:
- Identify the Atomic Number: Zinc has an atomic number of 30.
- Write the Neutral Electron Configuration: Use the Aufbau principle to write 1s² 2s² 2p⁶ 3s² 3p⁶ 4s² 3d¹⁰.
- Remove Electrons for Ionization: Remove the two 4s electrons to form Zn²⁺, resulting in 1s² 2s² 2p⁶ 3s² 3p⁶ 3d¹⁰.
✨ Note: Always follow the Aufbau principle and Hund’s rule when writing electron configurations.
Checklist for Understanding Zinc Ion Electron Configuration
- [ ] Identify zinc’s atomic number (30).
- [ ] Write the neutral electron configuration (1s² 2s² 2p⁶ 3s² 3p⁶ 4s² 3d¹⁰).
- [ ] Remove the 4s electrons to form Zn²⁺ (1s² 2s² 2p⁶ 3s² 3p⁶ 3d¹⁰).
- [ ] Understand the significance in biological and industrial applications.
Comparing Zinc Ion and Neutral Zinc Electron Configuration
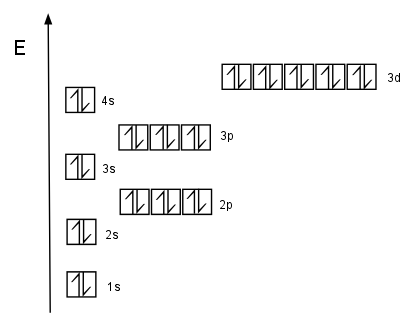
To better understand the differences, here’s a comparison table:
| State | Electron Configuration |
|---|---|
| Neutral Zinc (Zn) | 1s² 2s² 2p⁶ 3s² 3p⁶ 4s² 3d¹⁰ |
| Zinc Ion (Zn²⁺) | 1s² 2s² 2p⁶ 3s² 3p⁶ 3d¹⁰ |

This table highlights the removal of the 4s electrons in Zn²⁺, emphasizing the change in zinc ion electron configuration.
What is the electron configuration of Zn²⁺?
+The electron configuration of Zn²⁺ is 1s² 2s² 2p⁶ 3s² 3p⁶ 3d¹⁰.
Why does zinc lose 4s electrons first?
+Zinc loses 4s electrons first due to their higher energy level compared to 3d electrons, following the Aufbau principle.
What are the main applications of zinc ions?
+Zinc ions are used in biological systems, industrial processes like galvanization, and medical supplements.
In summary, the zinc ion electron configuration is 1s² 2s² 2p⁶ 3s² 3p⁶ 3d¹⁰, resulting from the loss of two 4s electrons. This configuration is fundamental to understanding zinc’s properties and applications in various fields. Whether for academic study or commercial use, mastering this concept is essential. For further exploration, consider researching zinc properties, electron configuration principles, or transition metal chemistry.