Bohr-Rutherford Diagram for Calcium: Visual Guide

<!DOCTYPE html>
Understanding the atomic structure of elements is crucial in chemistry and physics. The Bohr-Rutherford diagram for Calcium (Ca) provides a visual representation of its electron configuration, making it easier to grasp its atomic arrangement. This guide will walk you through creating and interpreting this diagram, catering to both informational and commercial-intent audiences.
What is a Bohr-Rutherford Diagram?

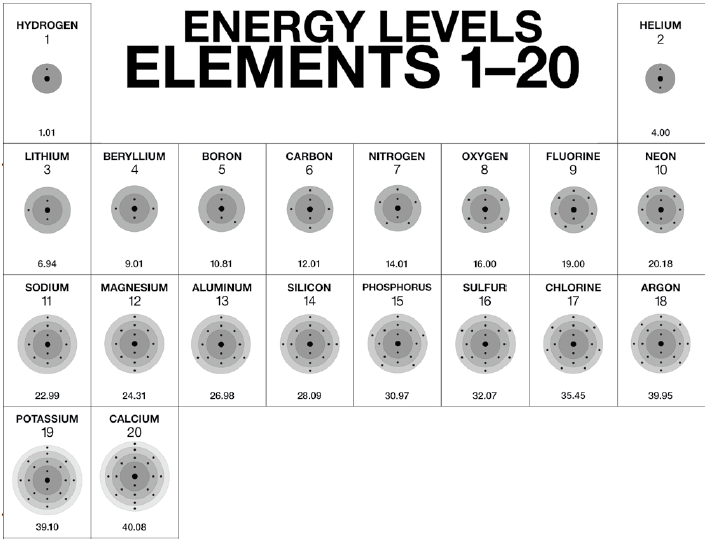
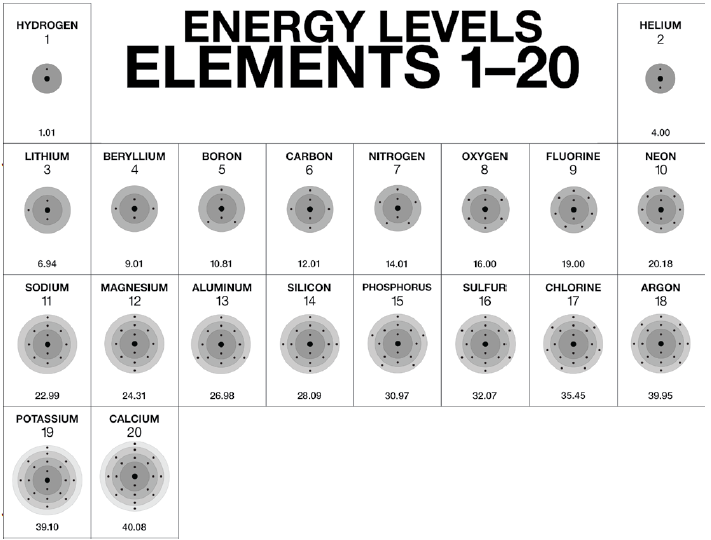
A Bohr-Rutherford diagram is a visual model of an atom, showing the nucleus and the arrangement of electrons in energy levels or shells. For Calcium (Ca), this diagram helps illustrate its 20 electrons distributed across different shells. Understanding this diagram is essential for students, educators, and professionals in fields like chemistry, physics, and materials science.
How to Draw the Bohr-Rutherford Diagram for Calcium

Drawing the Bohr-Rutherford diagram for Calcium involves a few straightforward steps:
- Step 1: Identify the Atomic Number – Calcium has an atomic number of 20, meaning it has 20 protons and 20 electrons.
- Step 2: Determine Electron Distribution – Use the 2-8-8-2 rule for electron configuration: 2 electrons in the first shell, 8 in the second, 8 in the third, and 2 in the fourth.
- Step 3: Draw the Nucleus – Represent the nucleus with a circle containing 20 protons and 20 neutrons.
- Step 4: Add Electron Shells – Draw concentric circles around the nucleus to represent the shells and place the electrons accordingly.
📌 Note: Ensure the diagram is clear and labeled for easy understanding.
Electron Configuration of Calcium

Calcium’s electron configuration is 1s² 2s² 2p⁶ 3s² 3p⁶ 4s². This configuration corresponds to the 2-8-8-2 distribution in the Bohr-Rutherford diagram. The outermost shell (4s²) determines Calcium’s chemical properties, making it a key element in biological processes and industrial applications.
Applications of Calcium’s Atomic Structure
Understanding Calcium’s atomic structure is vital for various applications:
- Biology – Calcium ions play a critical role in bone health and cellular signaling.
- Chemistry – Calcium compounds are used in construction, medicine, and food production.
- Industry – Calcium is essential in manufacturing alloys and removing impurities from metals.
Checklist for Creating a Bohr-Rutherford Diagram
Follow this checklist to ensure accuracy:
- Confirm the atomic number of the element (Calcium: 20).
- Determine the correct electron configuration (2-8-8-2 for Calcium).
- Draw the nucleus with the correct number of protons and neutrons.
- Add shells and place electrons according to the configuration.
- Label each shell and electron for clarity.
Mastering the Bohr-Rutherford diagram for Calcium enhances your understanding of atomic structures and their real-world applications. Whether you’re a student, educator, or professional, this visual guide is an invaluable resource for learning and teaching chemistry concepts, (atomic structure, electron configuration, Calcium properties, Bohr model, Rutherford diagram).
What is the electron configuration of Calcium?
+Calcium’s electron configuration is 1s² 2s² 2p⁶ 3s² 3p⁶ 4s², following the 2-8-8-2 distribution.
Why is Calcium important in biology?
+Calcium ions are essential for bone health, muscle function, and cellular signaling in biological systems.
How does the Bohr-Rutherford diagram differ from other atomic models?
+The Bohr-Rutherford diagram focuses on electron shells and energy levels, while other models like the quantum model describe electron behavior probabilistically.


